姜黄素对周围神经再生和S100表达的研究
李亚龙,李亚芳,赵一安,杨舒婷,刘国民,李秋菊
(1.吉林大学第二医院 骨科,吉林 长春130041;2.郑州大学第一附属医院 口腔科,河南 郑州450001;3.吉林大学第二医院 口腔科 ,吉林 长春130041)
姜黄素对周围神经再生和S100表达的研究
李亚龙1*,李亚芳2,赵一安3,杨舒婷3,刘国民1,李秋菊1*
(1.吉林大学第二医院 骨科,吉林 长春130041;2.郑州大学第一附属医院 口腔科,河南 郑州450001;3.吉林大学第二医院 口腔科 ,吉林 长春130041)
目的 探讨周围神经横断损伤后,姜黄素对周围神经再生的影响及其机制。方法 将BALB/c小鼠(N=200)随机分为正常对照组(0 mg/kg/天)、模型对照组(0 mg/kg/天)、低剂量组(10 mg/kg/天)、中剂量组(20 mg/kg/天)、高剂量组(40 mg/kg/天),每组40只,后4组建立坐骨神经横断损伤模型,姜黄素胃内给药1周。在姜黄素给药后的第1、2、4、8周,每组均进行电神经生理检测、免疫组织化学染色、Luxol Fast Blue (LFB)染色、实时定量聚合酶链反应(qPCR)、Western Blotting检测。结果 电神经生理检测显示,每组动作电位幅度和运动神经传导速度(MNCV)从第1周到第8周呈递增趋势,每周中剂量组和高剂量组的增幅大于低剂量组和模型对照组(P<0.05),高剂量组和低剂量组之间、低剂量组和模型对照组之间电生理特征差异无统计学意义(P>0.05);免疫组织化学染色显示,中剂量组和高剂量组的染色强度明显强于低剂量组和模型对照组;LFB染色显示,中剂量组和高剂量组重生有髓纤维的直径和数量明显多于低剂量组和模型对照组;qPCR和Western Blotting检测L4-6脊髓节段S100的表达水平,结果显示中剂量组和高剂量组S100的表达水平明显强于低剂量组和模型对照组(P<0.05)。结论 本研究结果表明,姜黄素可通过上调S100的表达,以浓度依赖性方式,促进横断周围神经的再生。本研究的结果有助于开发利用姜黄素治疗周围神经损伤。
姜黄素;周围神经损伤;坐骨神经;S100;神经再生
(ChinJLabDiagn,2017,21:1405)
姜黄素是姜科植物姜黄根茎的多酚提取物,呈黄色,在亚洲许多国家常用作烹饪调料[1]。有研究表明,姜黄素具有抗氧化、抗炎、抗病毒、抗癌等作用[2],因此也常用于治疗某些疾病[3,4]。姜黄素在中枢神经系统中具有广泛的靶点,通过调节HPA轴、影响脑神经递质、改善神经因子和神经再生、抑制神经元凋亡等多种途径,起到保护神经的作用[5-9],也可改善老年人的认知和情绪[10]。
姜黄素能够预防帕金森病和阿尔茨海默病[11-15],也能改善化疗药物诱发的神经病变[16,17]。外周神经损伤可导致相应神经元的死亡,姜黄素虽可促进坐骨神经挤压伤后的神经再生[18],但对周围神经横断损伤后功能恢复的影响及其机制的研究较少。
S100基因表达的S100蛋白含有两个钙结合位点,同时有高度保守的氨基酸序列[19],在细胞内和细胞外有多种功能[20],可诱导施万细胞的增殖促进神经再生[21]。本研究在此基础上,探讨了姜黄素在周围神经(坐骨神经)横断损伤BLAB/c小鼠模型中对周围神经再生的作用及其相关机制。
1 材料和方法
1.1 材料
姜黄素(St.Louis,MO,USA)购自Sigma-Aldrich,分子量368.39Da;雄性BALB/c小鼠(北京华阜康生物科技有限公司)200只,体重25±2 g,质量检测单位:中国医学科学院医学实验动物研究所,许可证号:SCXK(京)2014-0004,饲养于吉林大学基础医学院动物实验中心。动物实验方案已获得吉林大学动物伦理委员会批准。
1.2 动物管理
1.2.1 坐骨神经横断损伤模型的建立 将小鼠(N=200)分为正常对照组、模型对照组、低剂量姜黄素组(10 mg/kg/天)、中剂量姜黄素组(20 mg/kg/天)、高剂量姜黄素组(40 mg/kg/天),每组40只;后4组建立坐骨神经横断损伤模型:室温下向小鼠腹腔内注射3%氯胺酮(100 mg/kg/天)进行麻醉,俯卧位固定,在单侧后大腿做一长约2 cm的纵向切口,暴露梨状肌,将坐骨神经分离,在坐骨结节下方0.5 cm处将其切断,然后立即行显微手术将断端吻合,缝合肌肉和皮肤。
1.2.2 给药剂量分别取姜黄素50 mg、25 mg、12.5 mg、0 mg,各溶于25 μl的二甲基亚砜(DMSO)中,然后分别加0.9%NaCl溶液至50 ml,使DMSO在0.9%NaCl溶液中的最终体积浓度为0.05%,形成浓度为1 mg/ml的高剂量组、0.5 mg/ml的中剂量组、0.25 mg/ml的低剂量组、0 mg/ml的模型对照组。对各组小鼠进行灌胃治疗,1 ml/天,持续1周。未手术、无药物治疗的小鼠作为正常对照组;已手术,给予无姜黄素、含DMSO的0.9%NaCl溶液的小鼠作为模型组;已手术、给予姜黄素治疗的小鼠作为实验组。
1.3 电生理检测
姜黄素给药后的第1、2、4、8周,每组各取10只小鼠,分别在腹腔内注射3%氯胺酮(100 mg/kg/天)进行麻醉,室温下暴露坐骨神经,放置肌电图诱发电位仪(Keypoint○R,Medtronic A/C Inc,Skovlunde,Denmark),给予10 mA单电流刺激后,检测电极记录动作电位幅度和运动神经传导速度(MNCV=两个刺激电极之间的距离/动作电位潜伏期差异)。
1.4 L4-6脊髓节段取样
电生理检测完毕后,经后椎体中线切口打开椎管,将损伤的坐骨神经所连接的L4-6脊髓节段摘除,并立即储存在液氮中。
1.5 免疫组织化学染色
将姜黄素给药后第1、2、4、8周摘取的坐骨神经在10%中性甲醛中固定3天。将脊髓切片置于H2O2中浸泡10 min以阻断内源性过氧化物酶,并在柠檬酸钠溶液(0.01 mol/L,pH=6.0)中煮沸10 min。用0.01 mol/L的PBST(pH=7.4)冲洗切片,将切片用抗物种血清封闭处理15 min,加入兔抗小鼠S100多克隆抗体(1∶500,中国江苏省南京市碧云天生物技术研究所)后4℃条件下培养整夜。在37℃下加入生物酰化的山羊抗兔IgG(1∶1 000,中国湖北省武汉市博士德生物科技有限公司)处理20 min,然后加入链霉亲和素和Horseradish过氧化物酶处理20 min。利用3,3-二氨基联苯胺进行标记,棕色染色代表阳性蛋白。两名病理学专家利用显微镜(Olympus PM-10A0,中国北京市奥林巴斯有限公司)进行盲检,使用图像分析软件Image-Pro○RPlus 6.0 (MediaCybernetics Inc,Silver Spring,MD,USA)扫描染色区域来分析免疫组织化学光密度(IOD)。
1.6 Luxol Fast Blue(LFB)染色
将姜黄素给药后第1、2、4、8周摘取的坐骨神经在10%中性甲醛中固定3天,60℃下将坐骨神经切片在LFB溶液中染色12 h。将切片在95%乙醇中培养5 min,在0.05%碳酸锂溶液里浸泡15 s,再用70%乙醇和蒸馏水洗涤。将切片脱水,并清理干净,加盖盖玻片。每个切片中,随机选择5个区域在40×的倍数下进行显微镜观察。使用Image-Pro○RPlus 6.0图像分析软件计算有髓纤维的直径和数量。
1.7 实时定量聚合酶链反应(qPCR)
使用TRIzol试剂法,从姜黄素给药后第1、2、4、8周摘除的L4-6脊髓节段样品中提取出总RNA,以其为模板,通过逆转录酶合成cDNA,以cDNA为模板,通过qPCR技术,定量合成S100 mRNA。S100和甘油醛-3-磷酸脱氢酶(GAPDH)的引物(表1)由软件Beacon Designer 7(Premier Biosoft Inc,PaloAlto,CA,USA)设计,由生工生物工程(上海)股份有限公司合成。每个反应体系中,都有一对GAPDH引物作为内部对照,在95℃下反应30 s、58℃下反应60 s、72℃反应60 s,循环40次,获得循环阈值(Ct)。使用2-ΔΔCt法计算感兴趣区/GAPDH表达的mRNA。

表1 由Beacon Designer 7软件设计的S100和GAPDH引物
GAPDH:Glyceraldehyde-3-phosphate dehydrogenase.
1.8 Western Blotting检测
将姜黄素给药后第1、2、4、8周摘取的L4-6脊髓样品低温放在RIPA裂解缓冲液(碧云天生物技术研究所)中裂解。十二烷基硫酸钠-聚丙烯酰胺凝胶(12%)电泳分离蛋白质,将蛋白质电聚到聚偏二氟乙烯膜上。兔抗鼠S100多克隆抗体用含有1%牛血清白蛋白的PBS(碧云天生物技术研究所)按照1∶5 000稀释,将聚偏二氟乙烯膜置于其中,在4℃下培养整夜。再将其与Horseradish过氧化物酶标记的山羊抗兔IgG 在37℃下培养1 h,并用Western Blotting 3,3’-二氨基联苯胺试剂盒(碧云天生物技术研究所)对其染色,进行X射线荧光扫描和分析。感兴趣区与GAPDH蛋白质相对灰度值的比值表示蛋白质水平,用软件Image-Pro○RPlus 6.0对蛋白质水平进行分析。
1.9 统计分析
用SPSS17.0(SPSS Inc,Chicago,IL,USA)统计分析数据的平均值±SD,用单因素方差分析法和Dunnett-t检验比较组间差异,P<0.05为有统计学意义。
2 结果
2.1 电生理检测
为观测神经的功能情况,本研究用肌电图诱发电位仪(Keypoint○R,Medtronic A/C Inc,Skovlunde,Denmark)检测记录了坐骨神经动作电位幅度和MNCV(图1)。给予姜黄素治疗后,每组动作电位幅度和MNCV从第1周到第8周呈递增趋势。每周高剂量组和中剂量组的增幅大于低剂量组和模型对照组(P<0.05)。高剂量组第8周时动作电位幅度和MNCV增加到正常水平,分别为低剂量组的1.67倍和1.22倍。高剂量组和低剂量组之间、低剂量组和模型对照组之间电生理特征差异无统计学意义(P>0.05)。这些结果表明,姜黄素可促进小鼠坐骨神经损伤的功能恢复。
2.2 免疫组织化学染色
姜黄素给药后的第1、2、4、8周,对S100阳性蛋白的密度进行半定量检测,发现IOD值和阳性表达强度呈正相关(图2),同时,高剂量组的IOD值是低剂量组的1.79倍。从第4周开始,阳性细胞开始降低它的染色强度。每个时间段内,高剂量组和中剂量组的染色强度明显强于低剂量组和模型对照组。这些结果表明,姜黄素能够促进S100的表达。

A:Action potential amplitude;B:motor nerve conductive velocity.Data are expressed as the mean±SD,n=5.One-way analysis of variance and Dunnett’s test were used to analyze the differences among groups.*P< 0.05,vs.model group;#P<0.05,vs.low-dose curcumin group.
图1 姜黄素给药后第1、2、4、8周的动作电位幅度(mV)和运动神经传导速度(m/s)
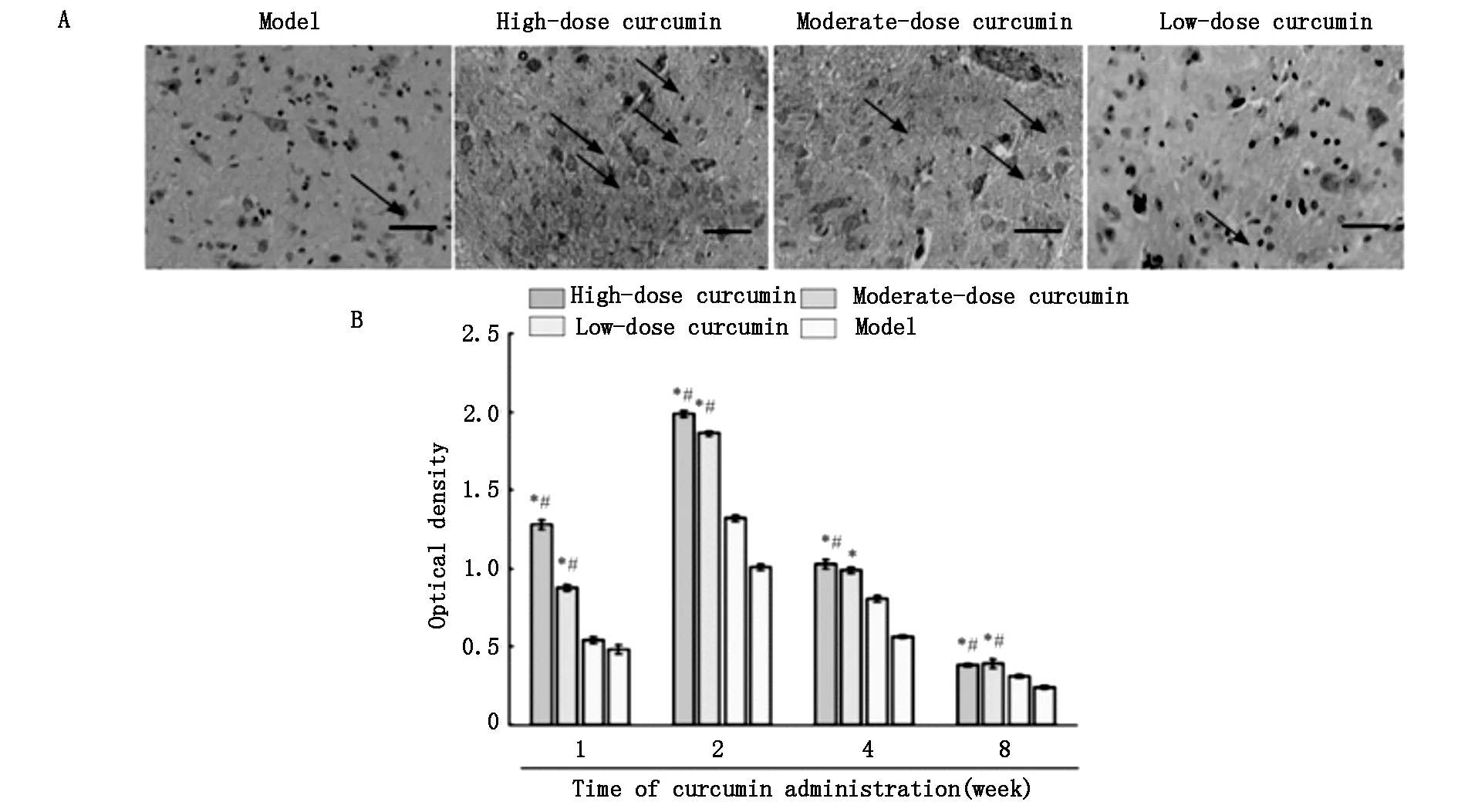
A:Immunohistochemical staining of S100 protein 2 weeks following curcumin administration at 40× magnification.Scale bar:200 μm.Arrows indicate positive immunoreactivity for S100,which is seen as fine brown particles in the cytoplasm.B: Staining intensity.The staining intensity in the high- and moderate-dose curcumin groups is stronger than that in the low-dose curcumin and model groups.Data are expressed as the mean± SD (n=5).One-way analysis of variance and Dunnett’s tests were used to analyze the difference among groups.*P<0.05,vs.model group;#P<0.05,vs.low-dose curcumin group.
图2 姜黄素给药8周后L4-6脊髓节段切片上的S100免疫反应
2.3 LFB染色
经LFB染色(图3)发现,姜黄素给药后的第1、2、4周,坐骨神经的髓鞘结构在各组间并无明显差异,第8周进行LFB染色后,轴突呈白色,髓磷脂呈蓝色。高剂量组和中剂量组中的髓磷脂形状规则,厚度均匀,边界清晰,髓鞘周围增生少;低剂量组中的髓磷脂规则不整,厚度不均,边界较清晰,有纤维结缔组织呈间质性增生;模型对照组髓磷脂情况最差。利用Image-Pro○RPlus 6.0测量有髓神经纤维数量和直径发现,高剂量组和中剂量组有髓神经纤维的数量和直径均大于低剂量组和模型对照组,高剂量组有髓神经纤维的数量和直径甚至分别是低剂量组的1.49倍和1.35倍。这些结果表明,高剂量组和低剂量组姜黄素可明显促进受损坐骨神经髓鞘的恢复。
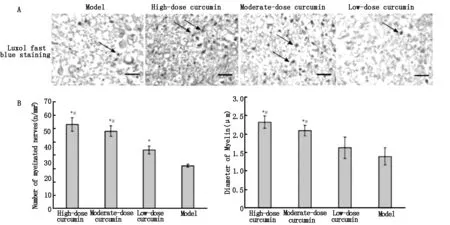
A:Luxol fast blue staining of transverse sections of sciatic nerve myelin.Images were obtained at 40× magnification;scale bar is 200 μm.Arrowsindicate the sciatic nerve myelin sheath dyed blue.Myelin in the high- and moderate-dose curcumin groups appears regular and uniform.However,myelin in the low-dose curcumin and model groups appears irregular and exhibits fibrous connective tissues.B:The number (n/mm2)and diameter (μm) of myelinated fibers in the high- and moderate-dose curcumin groups are larger than those in the low-dose curcumin andmodel groups.Data are expressed as the mean ±SD,n=5.One-way analysis of variance and Dunnett’s test were used to analyze the differencesamong groups.*P<0.05,vs.model group;#P<0.05,vs.low-dose curcumin group.
图3 姜黄素给药8周后,LFB染色显示的坐骨神经横断面有髓纤维的直径和数量
2.4 qPCR
在正常小鼠的坐骨神经中,几乎检测不到S100 mRNA。给予姜黄素治疗后,L4-6脊髓中S100 mRNA水平升高(图4)。第1周时,高剂量组和中剂量组S100 mRNA水平达到峰值,显著高于低剂量组和模型对照组,差异有统计学意义(P<0.05),其中高剂量组S100 mRNA水平比低剂量组约高2.15倍。低剂量组和模型对照组S100 mRNA水平则在第2周达到峰值。第8周时,S100 mRNA水平在各组间的差异无统计学意义(P>0.05)。这些结果表明,高剂量组和中剂量组姜黄素可明显促进S100表达。
2.5 Western-blotting检测
如图5所示,姜黄素给药后第2周,各组S100蛋白水平均上调,达到峰值。高剂量组和中剂量组明显高于低剂量组和模型组(P<0.05),其中高剂量组比低剂量组高1.4倍。这些结果表明,高剂量组和中剂量组对S100的表达有明显的促进作用。第8周时,各组间S100蛋白水平差异无统计学意义(P>0.05)。

Data are expressed as the mean±SD(n=5).One-way analysis of variance and Dunnett’s test were used to analyze the difference among groups.*P< 0.05,vs.model group;#P< 0.05,vs.low-dose curcumin group.
图4 姜黄素给药后1、2、4、8周L4-6脊髓节段中的S100 mRNA的表达
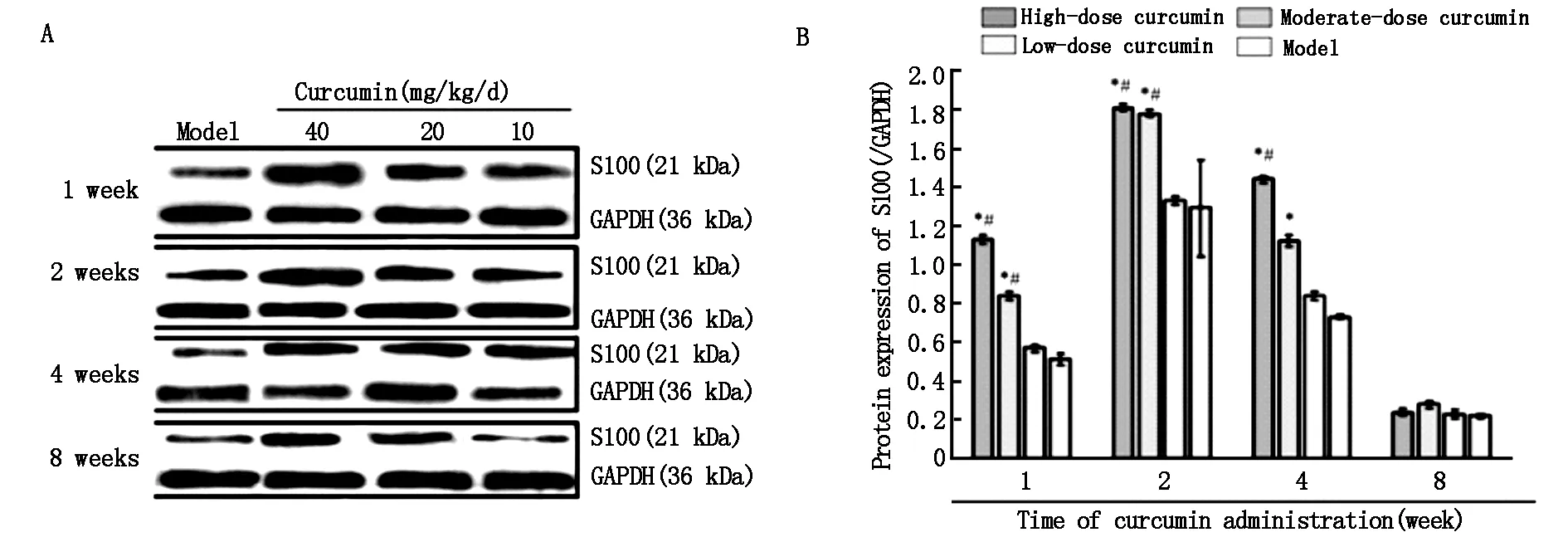
A:Western blot assay results for the S100 protein; B: Grayscale ratio of S100/GAPDH protein.S100 protein levels peak at the second week after curcumin administration in each curcumin group,and are dose-dependent for each time period.Data were expressed as the mean±SD(n=5).One-way analysis of variance and Dunnett’s test were used to analyze the difference among groups.*P<0.05,vs.model group;#P<0.05,vs.low-dose curcumin group.
图5 姜黄素给药后第1、2、4、8周L4-6脊髓节段中的S100 蛋白的表达
3 讨论
姜黄素对中枢神经系统具有保护作用[22-24],但其能否促进横断周围神经再生的相关研究较少,本研究对此进行了相关探究。用电神经生理检测观察坐骨神经的功能恢复情况,发现姜黄素以40 mg/kg/天(高剂量组)和20 mg/kg/天(中剂量组)的剂量给药一周后,横断的坐骨神经的功能有明显恢复。LFB染色实验显示,高剂量组和中剂量组再生坐骨神经髓鞘的数量和直径均大于低剂量组(10 mg/kg/天)和模型对照组,为姜黄素能够促进受损坐骨神经功能恢复提供了直接证据,同时表明姜黄素以浓度依赖性方式促进神经再生,也有相关研究证实了这一结果[18,25]。
本研究中,姜黄素20 mg/kg/天和40 mg/kg/天的给药剂量可有效促进小鼠受损神经的功能恢复,但当给药剂量为10 mg/kg/天时,小鼠受损坐骨神经功能恢复效果不明显,因此推测,姜黄素20 mg/kg/天的给药剂量可能是促进小鼠受损周围神经功能恢复的最低有效剂量。
为探究姜黄素促进坐骨神经再生的机制,本研究通过免疫组织化学染色、实时qPCR和Western blotting检测了S100 mRNA和蛋白质的表达水平。研究结果显示,S100 mRNA水平在姜黄素给药1周后达到峰值,S100蛋白质在姜黄素给药后第2周达到峰值,比S100 mRNA延迟1周,这是因为mRNA在蛋白质积累的同时也在快速降解。累积的S100蛋白质诱导坐骨神经Schwann细胞增殖,增殖的Schwann细胞促进坐骨神经的持续增生和功能恢复,因此S100常被作为神经细胞增殖的标志[26-28]。
综上所述,姜黄素以浓度依赖性方式,在20 mg/kg/天和40 mg/kg/天的给药剂量下,通过上调S100的表达,促进受损坐骨神经再生。本研究结果有助于开发利用姜黄素促进周围神经损伤的恢复。
[1]Mahmood K,Zia KM,Zuber M,et al.Recent developments in curcumin and curcumin based polymeric materials for biomedical applications:A review [J].Int J Biol Macromol,2015,81:877.
[3]Bimonte S,Barbieri A,Leongito M,et al.Curcumin AntiCancer Studies in Pancreatic Cancer[J].Nutrients,2016,8(7):433.
[4]Sarkar A,De R,Mukhopadhyay AK.Curcumin as a potential therapeutic candidate for Helicobacter pylori associated diseases[J].World J Gastroenterol,2016,22(9):2736.
[5]Daily JW,Yang M,Park S.Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials[J].J Med Food,2016,19(8):717.
[6]Jaisin Y,Thampithak A,Meesarapee B,et al.Curcumin I protects the dopaminergic cell line SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis[J].Neurosci Lett,2011,489(3):192.
[7]Patzkó A,Bai Y,Saporta MA,et al.Curcumin derivatives promote Schwann cell differentiation and improve neuropathy in R98C CMT1B mice[J].Brain,2012,135(12):3551.
[8]Cao H,Zheng JW,Li JJ,et al.Effects of curcumin on pain threshold and on the expression of nuclear factor κ B and CX3C receptor 1 after sciatic nerve chronic constrictive injury in rats[J].Chin J Integr Med,2014,20(11):850.
[9]Stankowska DL,Krishnamoorthy VR,Ellis DZ,et al.Neuroprotective effects of curcumin on endothelin-1 mediated cell death in hippocampal neurons[J].Nutr Neurosci,2015:1.
[10]Cox KH,Pipingas A,Scholey AB.Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population[J].J Psychopharmacol,2015,29(5):642.
[11]Yang J,Song S,Li J,et al.Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson's disease rat[J].Pathol Res Pract,2014,210(6):357.
[12]Venigalla M,Sonego S,Gyengesi E,et al.Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer's disease[J].Neurochem Int,2016,95:63.
[13]He Y,Wang P,Wei P,et al.Effects of curcumin on synapses in APPswe/PS1dE9 mice[J].Int J Immunopathol Pharmacol,2016,29(2):217.
[14]Song S,Nie Q,Li Z,et al.Curcumin?improves neurofunctions of 6-OHDA-induced parkinsonian rats[J].Pathol Res Pract,2016,212(4):247.
[15]Huang HC,Zheng BW,Guo Y,et al.Antioxidative and neuroprotective effects of curcumin in an Alzheimer's disease rat model co-treated with intracerebroventricular streptozotocin and subcutaneous D-galactose[J].J Alzheimers Dis,2016,52(3):899.
[16]Babu A,Prasanth KG,Balaji B.Effect of curcumin in mice model of vincristine-induced neuropathy [J].Pharm Biol,2015,53(6):838.
[17]Agthong S,Kaewsema A,Charoensub T.Curcumin ameliorates functional and structural abnormalities in cisplatin-induced neuropathy[J].Exp Neurobiol,2015,24(2):139.
[18]Ma J,Yu H,Liu J,et al.Curcumin promotes nerve regeneration and functional recovery after sciatic nerve crush injury in diabetic rats[J].Neurosci Lett,2016,610:139.
[19]Bresnick AR,Weber DJ,Zimmer DB.S100 proteins in cancer[J].Nat Rev Cancer,2015,15(2):96.
[20]Donato R,Cannon BR,Sorci G,et al.Functions of S100 proteins[J].Curr Mol Med,2013,13(1):24.
[21]Wolf H,Frantal S,Pajenda G,et al.Analysis of S100 calcium binding protein B serum levels in different types of traumatic intracranial lesions[J].J Neurotrauma,2015,32(1): 23.
[22]Srivastava P,Yadav RS,Chandravanshi LP,et al.Unraveling the mechanism of neuroprotection of curcumin in arsenic induced cholinergic dysfunctions in rats[J].Toxicol Appl Pharmacol,2014,279(3):428.
[23]Noorafshan A,Asadi-Golshan R,Abdollahifar MA,et al.Protective role of curcumin against sulfite-induced structural changes in rats' medial prefrontal cortex[J].Nutr Neurosci,2015,18(6):248.
[24]Ji MH,Qiu LL,Yang JJ,et al.Pre-administration of curcumin prevents neonatal sevoflurane exposure-induced neurobehavioral abnormalities in mice[J].Neurotoxicology,2015,46:155.
[25]Da Silva Morrone M,Schnorr CE,Behr GA,et al.Oral administration of curcumin relieves behavioral alterations and oxidative stress in the frontal cortex,hippocampus,and striatum of ovariectomized Wistar rats[J].J Nutr Biochem,2016,32:181.
[27]Liu Z,Jin YQ,Chen L,et al.Specific marker expression and cell state of Schwann cells during culture in vitro[J].PLoS One,2015,10(4):e0123278.
[28]Jiang X,Ma J,Wei Q,et al.Effect of Frankincense Extract on Nerve Recovery in the Rat Sciatic Nerve Damage Model[J].Evid Based Complement Alternat Med,2016,2016:3617216.
Effect of curcumin on peripheral nerve regeneration and S100 expression
LIYa-long1,LIYa-fang2,ZHAOYi-an3,etal.
(1.DepartmentofOrthopedics,theSecondHospitalofJilinUniversity,Changchun130041,China;2.DepartmentofStomatology,theFirstAffiliatedHospitalofZhengzhouUniversity,Zhengzhou450001,China;3.DepartmentofStomatology,theSecondHospitalofJilinUniversity,Changchun130041,China)
Objective To investigate the effect of curcumin on peripheral nerve regeneration after peripheral nerve transection and its mechanism.Methods BALB / c mice (N=200) were randomly divided into normal control group (0 mg/kg/day),model control group (0mg/kg/day),low dose group (10 mg/kg/day),middle dose group (20 mg/kg/day),high dose group (40 mg/kg/day),and each group of 40.BALB/c mice underwent complete sciatic nerve amputation,and followed by an immediate epineurium anastomosis.Mice were intragastrically administered curcumin at doses of 40 (high),20 (moderate),and 10 mg/kg/d (low) for 1 week.Following the 1st,2nd,4th and 8th week after curcumin administration were electroneurophysiological tests,immunohistochemical staining,Luxol Fast Blue (LFB) staining,real-time quantitative polymerase chain reaction (qPCR) and Western Blotting assay each group.Results Electroneurophysiological tests showed that the amplitude of each action potential and motor nerve conduction velocity (MNCV) were increasing from the first week to the eighth week.The increase of the middle dose group and the high dose group was higher than that of the low dose group and the model control group (P<0.05).There was no significant difference in electrophysiological characteristics between high dose group and low dose group or low dose group and model control group (P>0.05).Immunohistochemical staining showed that the staining intensity of the middle dose group and the high dose group was significantly higher than that of the low dose group and the model control group.Luxol Fast Blue (LFB) staining showed that the diameter of the myelinated fibers in the middle and high dose groups.QPCR and Western Blotting were used to detect the expression of S100 in L4-6 spinal cord segment.The results showed that the expression level of S100 in middle dose group and high dose group was significantly higher than that in low dose group and model control group (P<0.05).Conclusion The results of this study show that curcumin can promote the regeneration of peripheral nerves by up-regulating the expression of S100 in a concentration-dependent manner.The results of this study may help to develop the use of curcumin in the treatment of peripheral nerve injury.
Curcumin Peripheral nerve injury Sciatic nerve S100 Nerve regeneration
吉林省科技厅重大科技攻关项目(20150311038YY)
1007-4287(2017)08-1405-07
R651.3
A
李亚龙(1993-),男,河南省安阳市人,在读硕士研究生,主要从事神经再生方面的研究。
2017-03-25)
*通讯作者

