BsmI (rs1544410) and FokI (rs2228570) vitamin D receptor polymorphisms, smoking, and body mass index as risk factors of cutaneous malignant melanoma in northeast Italy
Sabina Cauci, Vincenzo Maione, Cinzia Buligan,3, Martina Linussio, Diego Serraino, Giuseppe Stinco,3
1Department of Medicine, University of Udine, Udine 33100, Italy;2Dermatology Clinic University Hospital of Brescia, Brescia 25123, Italy;3Dermatology Clinic, University Hospital of Udine, Udine 33100, Italy;4Unit of Cancer Epidemiology, CRO Aviano National Cancer Institute, Aviano 33081, Italy
BsmI (rs1544410) and FokI (rs2228570) vitamin D receptor polymorphisms, smoking, and body mass index as risk factors of cutaneous malignant melanoma in northeast Italy
Sabina Cauci1, Vincenzo Maione2, Cinzia Buligan1,3, Martina Linussio1, Diego Serraino4, Giuseppe Stinco1,3
1Department of Medicine, University of Udine, Udine 33100, Italy;2Dermatology Clinic University Hospital of Brescia, Brescia 25123, Italy;3Dermatology Clinic, University Hospital of Udine, Udine 33100, Italy;4Unit of Cancer Epidemiology, CRO Aviano National Cancer Institute, Aviano 33081, Italy
Objective: To investigate whether vitamin D receptor gene (VDR) BsmI-rs1544410 and FokI-rs2228570 polymorphisms, smoking duration, and body mass index (BMI) are risk factors for cutaneous melanoma, especially metastatic melanoma. Methods: We studied 120 cutaneous melanoma cases [68 stage I and II non-metastatic melanoma (NMetM) patients, plus 52 Stage III and IV metastatic melanoma (MetM) patients], and 120 matching healthy controls from northeast Italy. VDR polymorphisms were measured by restriction fragment length polymorphism analysis. Absence or presence of BsmI and FokI restriction sites was denoted by “B” and “F” or by “b” and “f,” respectively. Results: VDR-BsmI bb genotype was more frequent among MetM (32.7%) than among NMetM cases (13.2%), with odds ratio (OR)=3.18. Comparison of all melanoma patients vs healthy controls showed that the following biomarkers were at risk: ≥20 years of smoking (OR=2.43); ≥20 years of smoking combined with bb (OR=4.78), Bb+bb (OR=2.30), Ff (OR=3.04), and Ff+ff (OR=3.08); obesity (BMI>30 kg/m2) alone (OR=3.54); and obesity combined with Bb+bb (OR=3.52), Ff (OR=4.78), and Ff+ff (OR=6.56). Comparison of MetM vs NMetM patients revealed that the following biomarkers were at risk: ≥20 years of smoking (OR=2.39), ≥20 years of smoking combined with bb (OR=5.13), Bb+bb (OR=3.07), and Ff+ff (OR=2.66); and obesity combined with Bb+bb (OR=5.27), Ff (OR=6.28), and Ff+ff (OR=9.18). Triple combination of ≥20 years of smoking, obesity, and Bb+bb yielded OR=9.65 for melanoma patients vs healthy controls and OR=12.2 for MetM vs. NMetM patients. Conclusions: Risk factors for cutaneous MetM include two VDR polymorphisms combined with smoking duration and obesity. Results suggest gene-environment implications in melanoma susceptibility and severity. Future studies in larger cohorts and in subjects with different genetic background are warranted to extend our findings.
Vitamin D receptor; VDR polymorphism; cutaneous melanoma; metastatic melanoma; smoking; body mass index; obesity; skin cancer
Introduction
Melanoma continually presents increased incidence in all developed countries, particularly affecting fair-skinned individuals1-3. Malignant melanoma more frequently occurs in northern than in southern European countries3. Melanoma more frequently affects both sexes in Switzerland (European age standardized incidence rate 25.8/ 100,000/year) and Slovenia (20.6/100,000/year) than in Italy (13.4/100,000/year)4. Recent data in Italy5indicated a more than doubled prevalence of melanoma in northern than southern Italy, with central Italy presenting an intermediate value. Specifically, high incidence rates were recorded in Friuli-Venezia Giulia (FVG) region (19.6/100,000/year in men; 16.4/100,000/year in women) in northeast Italy6. This finding implies necessity for conducting geographically detailed studies regarding melanoma risk factors7. In the present study, we focused on inhabitants of the FVG region.
Critical environmental risk factors for melanoma include exposure to ultraviolet (UV) radiation, especially intermittent sun exposure and sunburns8,9. However, chronic and continuous UV ray exposure may yield protective effects9,10at least in part by activating synthesis of vitamin D, whose action is mediated by nuclear vitamin D receptor (VDR). Vitamin D-activated VDR may in turn up- or downregulate several hundreds of genes by binding to vitamin Dresponsive elements (VDREs), thus affecting several biological activities, such as calcium metabolism, immunity, detoxification, oxidative stress, cell proliferation, and differentiation9-12. Increasing evidence showed that vitamin D reduces risk of numerous types of cancer12. Thus, vitamin D endocrine system in studies concerning melanoma gained increasing attention10,13,14. Current studies and meta-analyses evaluated the role of the VDR gene (VDR) polymorphisms12-22. Nonetheless, VDR polymorphisms’ roles still require further study12,14,21.
The role of smoking in melanoma piqued interest of researchers23,24. Smoking is considered a risk factor for malignancies25. Paradoxically, several studies discovered inverse associations between smoking exposure and melanoma after controlling for potential confounding variables24,26,27. However, such protective effects are weak or insignificant28,29. Other studies did not confirm such association30,31or demonstrated tendencies toward smokingrelated increased risks10,32. Thus, pathophysiological pathways underlying the relationship of smoking and melanoma currently poses a challenge in melanoma research23,24.
Some studies on melanoma aimed to determine the role of body mass index (BMI) in occurrence of the disease9,10,33-35. However, limited research discusses combination of this biomarker with genetic traits.
Development in understanding of melanoma risk factors, genomics, and molecular pathogenesis may drive advances in precision medicine applied to melanoma2,13,14.
Human VDR gene is located in chromosome 12q12-q14 and comprises 11 exons and 11 introns18-22. Most clinical studies that explored association of VDR polymorphisms with diseases12,15,18,22focused on two VDR single-nucleotide polymorphisms (SNPs), namely, BsmI-rs1544410 G>A located in intron 8 and FokI-rs2228570 C>T located in exon 2. These two polymorphisms show no linkage disequilibrium (LD)18,22.
We explored VDR BsmI-rs1544410 and FokI-rs2228570 SNPs separately, and their association with lifestyle factors, particularly smoking duration and BMI of patients with cutaneous malignant melanomas, specifically those with metastatic melanoma (MetM) vs. non-metastatic melanoma (NMetM) and vs. healthy controls.
Patients and methods
Population
Enrollment and clinical visits of all study participants were performed at Dermatology Clinic, University Hospital of Udine. Diagnostic procedures were carried out according to routine protocols. The Udine Institutional Ethical Committee approved the study protocol, which was conducted according to the Declaration of Helsinki. All participants were alive during enrollment in the study and signed a written informed consent.
Using a case-control design, the study consecutively enrolled 120 (65 males and 55 females, age range of 31–84 years) unrelated patients (hospitalized or outpatients) with documented cutaneous melanoma diagnosis and 120 (65 males and 55 females, age range of 31–84 years) asymptomatic healthy controls, which were matched for gender, ancestry, and age with melanoma cases. Inclusion criteria for both melanoma cases and healthy controls were as follows: resident in FVG region, at least two grandparents born in FVG region (or Austro-Hungarian territory before World War I), and two grandparents, at the most, with central or southern Italian ancestry. Exclusion criteria for controls included the following: any kind of lifelong malignant or benign tumor, first-grade relatives with history of melanoma, and major chronic diseases, such as autoimmune diseases, type 1 diabetes, and thyroid diseases.
Melanoma was diagnosed using immunohistological findings obtained after surgical excision of nevi with clinical and dermoscopic characteristics suggesting presence of malignancy. Classification of melanoma stages was performed by clinical/histological/radiological findings, as described in final version of 2009 AJCC36. Inclusion criteria for case patients comprised cutaneous melanomas that were more severe than in situ only and with a Clark-grade invasion over I. For patients with multiple melanomas, the major melanoma characteristics were accounted for in study analyses according to histological assessment of major primary tumor (T) grading.
Each participant answered a questionnaire, which was used to collect data on demographic characteristics, medical and family history of melanoma, smoking habits, alimentary habits, and history of sunburns. Phototype was assessed by Fitzpatrick criteria37. BMI was determined by weight (kg) divided by squared height (m2); BMI>30 kg/m2was considered as an indicator of obesity.
Genetic analysis ofVDRpolymorphisms
VDR-BsmI G>A and VDR-FokI C>T polymorphisms were determined, as described in Refs.38,39, after extraction of genomic DNA from ethylenediaminetetraacetic-acid-treated venous blood samples40. Genotypes were designated according to absence/presence of the BsmI or FokI enzymerestriction site by a capital letter B allele, or F allele for absence, and by a lowercase letter b allele, or f allele for presence, respectively41. FokI and BsmI polymorphisms of VDR were studied using previously tested primers38-40, which were used to amplify appropriate DNA fragments. The following primers were specifically used: FokI-forward (5′-AGC TGG CCC TGG CAC TGA CTC TGC TCT-3′) and FokI-reverse (5′-ATG GAA ACA CCT TGC TTC TTC TCC CTC-3′); BsmI-forward (5′-CAA CCA AGA CTA CAA GTA CCG CGT CAG TGA-3′) and BsmI-reverse (5′-AAC CAG CGG GAA GAG GTC AAG GG-3′) primers. FokI enzyme (Euroclone, Milano, Italy) digestion of amplified 265 bp DNA fragment resulted in two 196 and 69 bp fragments in the presence of f allele40. To analyze BsmI polymorphism, the resulting amplified 825 bp fragment was digested with BsmI restriction enzyme (Euroclone, Milano, Italy), generating two fragments of 650 and 175 bp in the presence of b allele39.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and Mann-Whitney U test was performed for comparison. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for categorical variables, and P values for two-sided Pearson’s Chi-squared or Fisher’s exact test were reported as appropriate. Logistic regression was used to evaluate effects of confounders by obtaining adjusted ORs and CIs. Five different combinations of confounders were tested. Adjusted analysis included conventional risk factors: (1) gender and age; (2) gender, age, phototype 1+2, total number of body nevi>50, and number of lifelong sunburns>10. To compare MetM and NMetM, adjusted analyses included indicators that resulted in risk of metastasis development: (3) trunk location, Breslow’s thickness, ulceration, mitosis>1, absence of tumor-infiltrating lymphocytes (TILs), and epithelioid variant; (4) ≥20 years of smoking; and (5) BMI>30 kg/m2(i.e., obesity). Adjusted analysis of type 3 confounders involved factors associated (according to our findings) with ≥20 years of smoking. These factors included TIL absence, ulceration and obesity. Thus, to avoid overcorrection, combined categorical variables, including smoking and obesity, were not adjusted for type 3 confounders.
Tests for deviations from Hardy-Weinberg equilibrium (HWE) were separately performed using chi-square distribution for each SNP39,40. LD between SNPs was determined as described by Colombini et al.39
A two-sided value of P<0.05 was considered significant, and P≤0.10 indicates tendency to be significant. Statistical software SPSS for Windows (SPSS Inc., Chicago, IL, USA) was used.
Results
All 240 (120 cutaneous melanoma patients + 120 healthy controls) study subjects were Italian white residents in the FVG region.
Primary clinical characteristics of melanoma patients
As reported in Table 1, we examined in detail differences between MetM and NMetM patients to also identify appropriate variables to be included as confounders in subsequent multivariate analyses. Frequency of young (<40 years old) or old (≥60 years old) melanoma patients at study enrolment did not differ between MetM and NMetM groups (mean age comparisons reported in Table 2). Mean age at melanoma diagnosis reached 53.1±13.26 years. Mean time for melanoma diagnosis totaled 6.5±3.58 years and did not differ between MetM and NMetM patients.
The majority of 68 NMetM patients were in stage I (70.6%), whereas the majority of 52 MetM patients were in stage III (65.4%). Location in the trunk (OR=0.35) and superficial spreading (OR=0.31) showed protective effects for MetM patients vs. NMetM patients. Mean Breslow’s thickness doubled in MetM cases vs. NMetM cases (2.8±1.74 vs. 1.4±1.34 mm, P<0.001). Specifically, a Breslow’s thickness≤0.75 mm had protective effects (OR=0.06), whereas thickness ≥4.01 mm was risky (OR=9.90) for MetM vs. NMetM cases. Some biomarkers were more frequently observed in MetM than in NMetM patients. These biomarkers included Clark IV invasion (OR=4.38), ulceration (OR=3.79), mitosis >1 (OR=3.77), TIL absence (OR=2.20), and epithelioid variant (OR=2.98).
Obesity and smoking history
By comparing obese and non-obese melanoma patients, we observed that non-brisk TIL cases were less frequent in obese (1/16, 6.25%) than in non-obese (40/104, 38.5%) patients, with OR=0.11, 95% CI=0.01–0.84, and P=0.011. By contrast, TIL absence was more frequent in obese (10/16, 62.5%) than in non-obese (31/104, 29.8%) melanoma patients, resulting in OR=3.92, 95% CI=1.31–11.7, and P=0.010.
Similar findings were observed by comparing melanoma patients who smoked ≥20 years vs. the remaining melanoma patients. Frequency of non-brisk TIL cases was lower in ≥20-year smokers (7/36, 19.4%) than other melanoma patients (34/84, 40.5%), yielding OR=0.35, 95% CI=0.14–0.90, and P=0.026. By contrast, TIL absence was more frequent in ≥20-year smokers (17/36, 47.2%) than in other melanoma patients (24/84, 28.6%), with OR=2.24, 95% CI=1.00–5.02, and P=0.048. By comparing ≥20-year smokers with the remaining melanoma patients, we detected significant findings for males (OR=4.45, 95% CI=1.81–10.9, P=0.001), stage III melanoma (OR=2.44, 95% CI=1.06–5.64, P=0.034), and ulceration (OR=2.50, 95% CI=1.12–5.56, P=0.023).
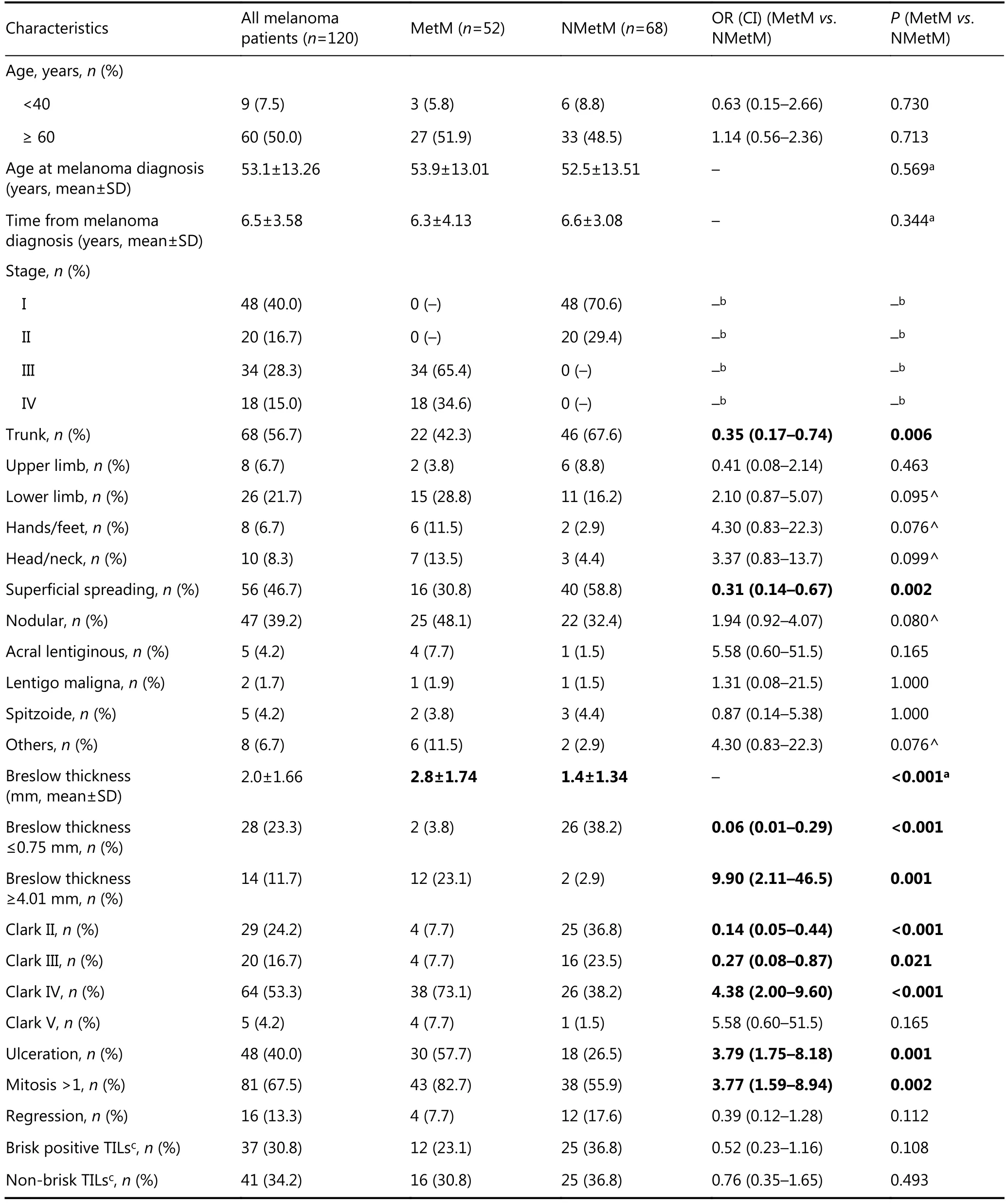
Table 1 Clinical characteristics of 120 consecutively enrolled melanoma patients and comparison between the two subgroups of 52 MetM and 68 NMetM patients

Continued

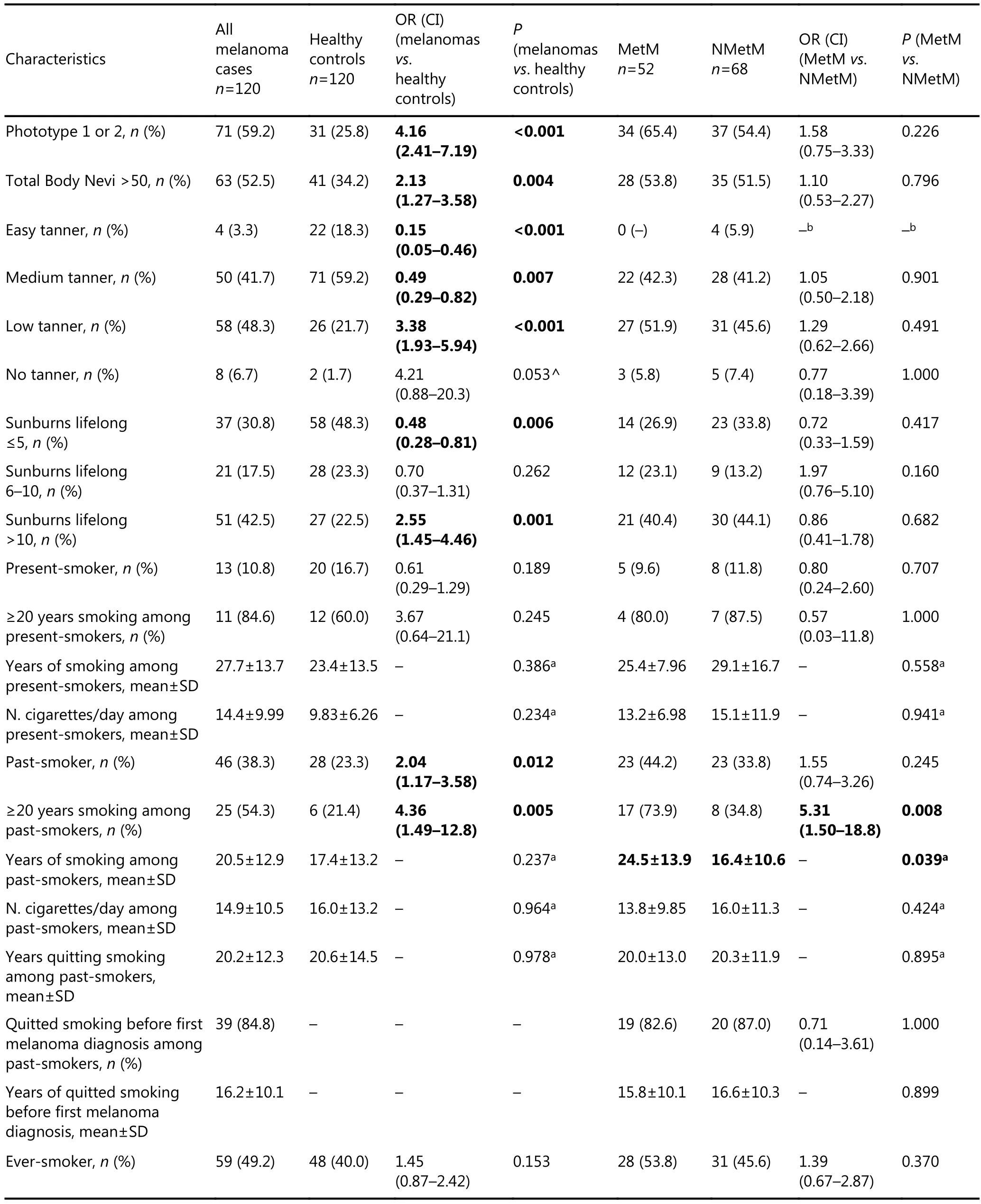
Table 2 Comparison of demographic characteristics of 120 melanoma patients and 120 healthy controls and comparison between the two subgroups of 52 MetM and 68 NMetM patients
Continued

Continued
Comparison of demographic, behavioral, and environmental variables (Table 2)
Melanoma patients yielded higher mean BMI than healthy controls (P=0.010), and the number of obese subjects was over threefold higher (OR=3.54) among melanoma patients than among healthy controls.
Melanoma patients more frequently presented phototype 1+2 (OR=4.16), total number of body nevi>50 (OR=2.13), lifelong sunburns>10 (OR=2.55), and were more frequently low tanners (OR=3.38) than healthy controls. MetM patients did not differ from NMetM patients in terms of these characteristics.
Past smokers were twofold more frequent among melanoma patients than healthy controls. Among past smokers smoking for ≥20 years was considerably more frequent in melanoma patients (OR=4.36) than healthy controls and in MetM (OR=5.31) than NMetM patients. Past smokers with MetM showed higher average number of smoking years than NMetM patients (24.5±13.9 vs. 16.4±10.6 years; P=0.039). Among past smokers, melanoma patients quitted smoking for an average of 16.2±10.1 years before melanoma diagnosis, and differences were not observed between MetM and NMetM patients.
Twenty or more years of smoking among lifelong smokers and among all study subjects was a risk factor for melanoma patients vs. healthy controls (OR=2.61 and OR=2.43, respectively) and for MetM vs. NMetM patients (OR=3.20 and OR=2.39, respectively).
The majority of melanoma patients and healthy controls were daily coffee drinkers; no difference was noted among groups even when considering those who consumed over three cups of coffee per day.
Unadjusted comparisons ofVDR-BsmI andVDR-FokI genotypes alone or combined with smoking and obesity (Table 3)
VDR-BsmI and VDR-FokI genotypes were in HWE in healthy controls and in melanoma patients. As expected, the two SNPs were not in LD.
Homozygous bb genotype was more frequent among MetM than among NMetM patients (OR=3.18). Intriguingly, bb frequency was lower in NMetM patients than in healthy controls (OR=0.40). Genotype bb combined with ≥20 years of smoking was more frequent among all melanoma patients than healthy controls (OR=4.78), in MetM than NMetM patients (OR=5.13), and in MetM patients than healthy controls (OR=9.18). The same profile was observed for Bb+bb (b allele carriers) plus ≥20 years of smoking. Carriersof b allele (Bb+bb) who were obese showed increased risk for all melanomas (OR=3.52 for melanoma patients vs. healthy controls) and MetM (OR=5.27 for MetM vs. healthy controls). Notably, the combination of three parameters, i.e., Bb+bb genotype plus ≥20 years of smoking plus obesity yielded high ORs for all melanoma patients vs. healthy controls (OR=9.65), MetM vs. NMetM patients (OR=12.2), and MetM patients vs. healthy controls (OR=21.6).
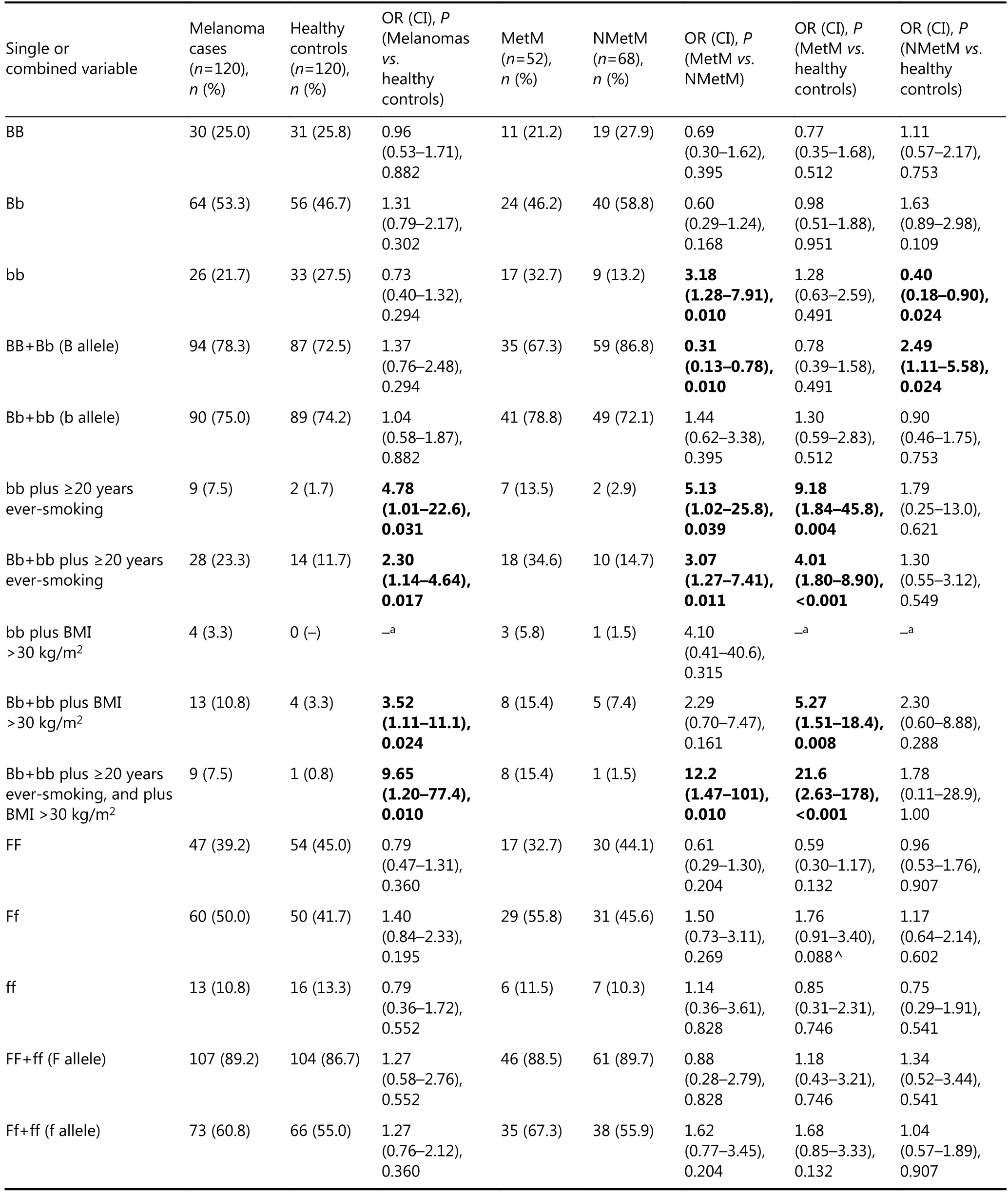
Table 3 VDR-BsmI and VDR-FokI genotypes alone or combined with smoking duration and obesity compared between 120 melanoma cases and 120 healthy controls.

Continued
As shown in Table 3, VDR-FokI genotype FF, Ff, and ff frequencies did not differ among groups. However, heterozygous Ff had a tendency to be more frequent among MetM patients than healthy controls (OR=1.76; P=0.088). Notably, Ff genotype combined with ≥20 years of smoking acted as risk factor for all melanoma patients (OR=3.04 for melanoma patients vs. healthy controls) and MetM patients (OR=4.84 for MetM patients vs. healthy controls). Carriers of f allele (i.e., Ff+ff) combined with ≥20 years of smoking posed risk for all melanoma patients (OR=3.08 for melanoma patients vs. healthy controls) and for MetM (OR=5.00 for MetM patients vs. healthy controls, and OR=2.66 for MetM vs. NMetM patients). Ff genotype combined with obesity exhibited OR=4.78 for all melanoma patients vs. healthy controls and OR=6.28 for MetM patients vs. healthy controls. Finally, obese carriers of Ff+ff presented an increased risk for all melanomas (OR=6.56 for all melanoma patients vs. healthy controls) and for MetM (OR=9.18 for MetM patients vs. healthy controls). Notably, only 6 out of 240 study subjects showed the triple combination of Ff+ff genotype, ≥20 years of smoking, and obesity, and they were all MetM patients.
Comparisons ofVDR-BsmI andVDR-FokI genotypes, smoking, and obesity alone or their combinations (Table 4)
As shown in Table 4, by comparing all 120 melanoma cases vs. 120 healthy controls, four variables including the parameter ≥20 years of ever smoking among all subjects were significant after multivariate analysis of type 1 confounders (including gender and age): ≥20 years of smoking alone (OR=2.19), or plus Bb+bb (OR=2.08), plus Ff (OR=2.77), and plus Ff+ff (OR=2.86) genotype. However, all those differences became not significant adding more confounding factors by analysis of type 2 confounders. Multivariate analysis of type 2 confounders revealed that five variables, including obesity, were all risk factors for melanoma patients vs. healthy controls, and they were as follows: BMI>30 kg/m2alone (OR=5.28), or plus Bb+bb (OR=4.35), plus Ff (OR=6.91), and plus Ff+ff (OR=8.89) genotype, and triple combination of Bb+bb, ≥20 years of smoking, and obesity (OR=12.0).

?
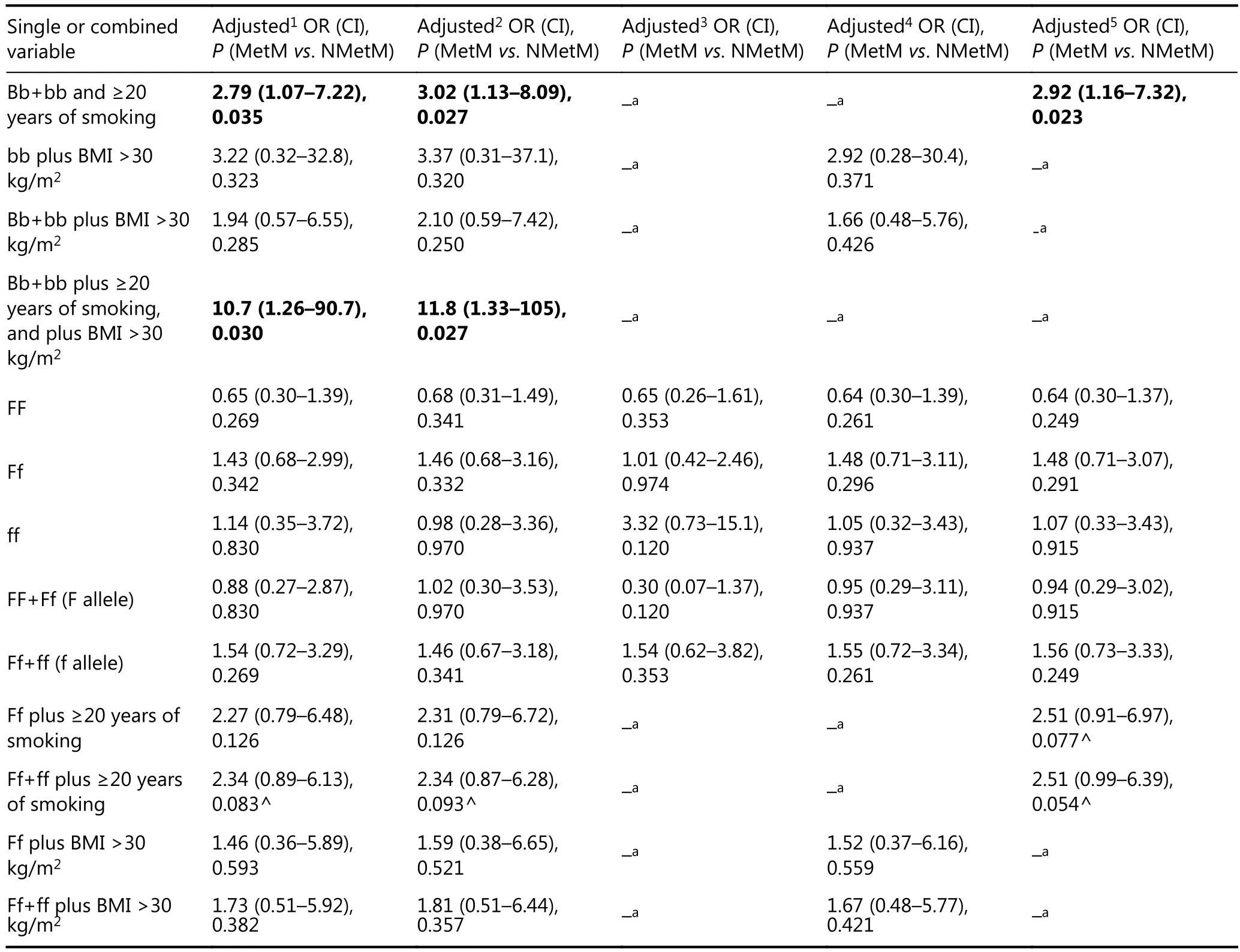
Comparison of MetM patients vs healthy controls revealedthat combination of Bb+bb and ≥20 years of smoking was significant (OR=3.29) after adjustment of type 1 confounders, but became a tendency after adjustment of type 2 confounders. By contrast, eight other variables were significant after both multivariate analyses of types 1 and 2 confounders. Specifically, by analysis of type 2 confounders, the following significant findings were observed: ≥20 years of smoking alone (OR=2.46) or combined with bb (OR=6.99), Ff (OR=3.69), and Ff+ff (OR=3.92) genotype; and obesity alone (OR=7.74) or combined with Bb+bb (OR=6.55), Ff (OR=14.6), and Ff+ff (OR=17.8) genotype. Notably, triple combination of Bb+bb, ≥20 years of smoking, and obesity resulted in type 2-adjusted OR=22.7 for MetM patients vs healthy controls.
Adjusted comparisons of type 2 confounders among NMetM patients vs healthy controls revealed risk effects of obesity (OR=3.69), Bb (OR=2.15), and BB+Bb (OR=3.30) genotypes.
Comparisons ofVDR-BsmI andVDR-FokI genotypes, smoking, and obesity alone or their combinations (Table 5)
Table 5 illustrates comparison of MetM vs NMetM patients by adjusted analyses of types 1 to 5 confounders. Smoking duration of ≥20 years is a significant risk factor for MetM vs. NMetM patients (OR=2.26, 95% of CI=1.00–5.10, P=0.050) after adjustment for obesity (type 5 confounder). However, this risk factor became a tendency after extensive adjustments (types 1 and 2 confounders). Notably, bb genotype showed consistent risky adjusted OR=3 for MetM vs. NMetM after multivariate analysis of types 1 to 5 confounders. Consequently, carriage of B allele (i.e., BB+Bb) resulted in protective effects with respect to MetM. Significant threefold increased risk for MetM vs. NMetM cases was observed for the combination of Bb+bb and ≥20 years of smoking after adjustments for types 1, 2, and 5 confounders. Finally, triple combination of Bb+bb, ≥20 years of smoking, and obesity showed high types 1 and 2-adjusted ORs (OR=10.7 and OR=11.8, respectively), thus attesting for gene-behavioral effects among MetM patients.
Discussion
Our study was carried out under the context of precision medicine approach for disease treatment and prevention, which considers individual variability in genes, environment, and lifestyles42.
VDR-BsmI polymorphism
We observed similar general distribution of VDR-BsmI genotypes (BB 25.0%, Bb 53.3%, bb 21.7%) among allmelanoma patients and healthy controls (BB 25.8%, Bb 46.7%, bb 27.5%), showing agreement with other casecontrol investigations16,20. Notably, a threefold higher frequency of bb genotype was observed in MetM (32.7%) compared with NMetM cases (13.2%); this value ranged from significant crude OR=3.18 to adjusted ORs ranging from 3.06 to 3.42 after considering several confounders. We observed that B carriers (BB+Bb) were at reduced risk comparing MetM vs. NMetM cases. However, B carriers were at increased risk when comparing NMetM vs. healthy controls. Paradoxically, by comparison with healthy controls, carriage of bb genotype posed risk to MetM, but was protective for NMetM cases. In this study, distributions of genotypes in melanoma patients were similar with respect to Bb frequencies of those observed in central Italy by Santonocito et al.15in 101 melanoma patients (BB 9.9%, Bb 53.5%, bb 36.6%). The study indicated increased frequencies of Bb and bb genotypes in melanoma patients compared with healthy controls (BB 23.8%, Bb 50.5%, bb 25.7%) and demonstrated an association between VDR-BsmI bb genotype and increased Breslow’s thickness15, a parameter that is consistently associated with metastasis and poor prognosis9. A meta-analysis17showed that BsmI B allele is associated with reduced melanoma risk with OR=0.81 and 95% CI=0.72–0.92. A large-scale study of incidence of multiple primary melanoma revealed distribution of VDRBsmI, with values of BB 18.9%, Bb 46.8%, and bb 34.2%, among patients with multiple primary melanomas and BB15.3%, Bb 47.7%, and bb 37.0% among patients with single primary melanoma19. A recent meta-analysis22reported a 15% decrease in melanoma risk (pooled OR=0.85, 95% CI=0.76–0.94) for individuals with BB or Bb genotype compared with subjects featuring bb genotype.

Table 5 Association of ≥20 years of smoking, obesity, VDR-BsmI genotype, and VDR-FokI genotype as single or combined variables with MetM (n=52) vs. NMetM (n=68), as evaluated by adjusted1,2,3,4,5OR (CI)
Continued
In our study, bb genotype combined with ≥20 years of smoking yielded adjusted OR=7 for MetM patients vs healthy controls. Bb+bb (i.e., b allele) genotype combined with obesity showed adjusted ORs from 4 to 7 for MetM patients vs healthy controls.
Functional effect of VDR-BsmI polymorphism remains unclear39,41,43. This SNP is located in an intron sequence at the 3’ end of VDR gene. Thus, VDR-BsmI polymorphism cannot directly change the protein sequence of the VDR receptor. Some studies suggested that this SNP can influence VDR-mRNA expression, thereby affecting its stability43. BsmI site may be in LD with other truly relevant SNPs in VDR or other genes14,15,44.
VDR-FokI polymorphism
In our study, VDR-FokI genotypes (FF 39.2%, Ff 50.0%, and ff 10.8% in melanoma cases vs. FF 45.0%, Ff 41.7%, and ff 13.3% in healthy controls) were not associated with melanoma, and this result agrees with results of a recent meta-analysis21. However, we noted an increased risk for heterozygous Ff carriers when we compared MetM vs. NMetM cases. A Serbian study showed that compared with ff genotype, Ff and FF were associated with increased melanoma risk (OR=3.03, P=0.003; OR=9.28, P<0.001, respectively)45. In general, inconsistent findings were reported for association of VDR-FokI polymorphism with melanoma19,46. In one meta-analysis20, FokI polymorphism was associated with an overall significantly increased risk of skin cancer (Ff vs. FF: OR=1.20, 95% CI=1.01–1.44; ff vs. FF: OR=1.41, 95% CI=1.08–1.84; Ff+ff vs. FF: OR=1.26, 95% CI=1.04–1.53). Another meta-analysis22claimed that f allele carriers showed an 18% (pooled OR=1.18, 95% CI=1.07–1.29) increased risk for melanoma compared with FF homozygotes. Notably, in our study, Ff+ff (f allele carriers), when combined with ≥20 years of smoking or with obesity, exhibited adjusted OR=4 and ORs from 8 to 18, respectively, for MetM patients vs. healthy controls. The f allele codes for a 427 amino acids long VDR protein, and it is considered less effective than the protein receptor coded by F allele (424 amino acids long)40,41,43.
Smoking
Our study highlighted the crucial role of smoking duration in susceptibility to cutaneous melanoma and MetM. Pastsmoking for ≥20 years resulted in fourfold risk factor for melanoma development with respect to healthy controls (OR=4.36) and fivefold risk factor for development of MetM with respect to NMetM (OR=5.31), whereas ≥20 years of smoking ever in life yielded OR=2.43 and OR=2.39, respectively. We also observed that ≥20 years of ever in life smoking combined with certain genetic traits, specifically, with bb, Bb+bb (b allele carriers), Ff, and Ff+ff (f allele carriers) are associated with significant crude ORs ranging from 4 to 9 for MetM cases vs healthy controls. Thus, smoke effects in melanoma can be modulated by VDR activity and by the pleiotropic vitamin D endocrine system11-13,44. Further studies are necessary to substantiate this significant and complex issue10,11,44.
Despite the large number of studies23,24,26-28, results on association of smoking with melanoma still present inconsistencies23. Some authors demonstrated risk effects of smoking in melanoma10,23. Using multivariate analysis (adjusted for age, sex, site of primary melanoma, and Breslow’s thickness), a recent study by Newton-Bishop et al.10revealed that smoking duration at diagnosis (hazard ratio=1.11, 95% CI=1.03–1.20, P=0.009) is associated with risk of death from melanoma; and that lower vitamin D levels and smoking are associated with ulceration (a well-known poor prognostic factor) of primary melanomas and poor melanoma-specific survival10. We also noted positive association of ≥20 years of smoking with ulceration among melanoma patients. We similarly observed association of ≥20 years of smoking with TIL absence, a finding that predisposes in our and other studies to metastatic melanoma8,9. Conversely, other authors showed inverse relationship of smoking with melanoma26-28. Multiple potential confounders and biases can explain those protective associations of smoking23. Our present findings suggest that effects of smoking duration may be modulated by specific genetic traits.
In our study, past smokers among melanoma cases were twofold more frequent than among healthy controls. Our findings show association of ≥20 years of smoking with increased risk of melanoma, indicating the need for detailed assessment of lifelong smoke duration. We observed that among past smokers, MetM patients smoked approximately 8 years longer than NMetM patients (24.5±13.9 vs. 16.4±10.6 years). Notably, we demonstrated that ≥20 years of smoking serves as a two- to fivefold risk factor for MetM compared with NMetM patients. Among study participants, over 80% of past smokers with melanoma quit smoking before cancer diagnosis, with an average of 16 years before melanomadevelopment. This finding implies that exposure to smoke carcinogens requires long periods to induce melanoma onset and/or metastatic stage. Smoking effects long after smoking discontinuation provide intriguing evidence, which implies that some irreversible damages occur several years before first melanoma diagnosis. Long-lasting variations induced by smoking may include epigenetic changes in specific genes that can remain, for example, differentially methylated after smoking cessation (up to 22 years, as demonstrated by Ambatipudi et al.47and/or body accumulation of substances, such as heavy metals, including radionuclides Lead-210 and Polonium-21048,49. Explanation for such smoking phenomena require future detailed biological research and human studies. A recent study on melanoma cells observed a role for epigenetic mechanisms in VDR-miRNAs regulation50.
In our study, ≥20 years of smoking combined with carriage of b allele (Bb+bb) showed adjusted OR=3 for MetM vs. NMetM patients after extensive multivariate analyses. This issue warrants further large-scale studies.
Coffee
We did not observe any significant findings in terms of coffee consumption. Thus, our data do not confirm protective effects of coffee consumption, as observed by other researchers29.
Obesity
In our study, obesity presented an almost fourfold risk factor for melanoma susceptibility (OR=3.54), similar to previous population studies on malignant melanoma26,33. Obesity yielded an adjusted OR=5 for all melanoma patients vs. healthy controls and adjusted OR=8 for MetM patients vs. healthy controls. Obesity combined with Ff or Ff+ff exhibited high adjusted OR=15 and OR=18, respectively, for MetM patients vs. healthy controls. BMI is extensively evaluated in relation to several cancer types51. A large-cohort Italian study demonstrated that BMI≥25 kg/m2is associated with Breslow’s thickness>1 mm among melanoma patients9.
We are the first research group to assess the role of combination of obesity with specific VDR genetic traits in cutaneous melanoma. Interpretation of the association of obesity with melanoma may feature a biological rationale. Newton-Bishop et al.10hypothesized that inflammation associated with obesity can influence outcome of melanoma. Some evidence also showed the genetic link between obesity and pigmentation or hair color52.
Triple combination ofVDRgenetic traits, smoking, and obesity
In our study, the highest ORs were observed after combination of a VDR genetic trait (b allele carriers) and two lifestyle parameters, i.e., Bb+bb plus ≥20 years of smoking and plus obesity by comparing all melanoma patients vs. healthy controls (OR=9.65), MetM patients vs. healthy controls (OR=21.6), and MetM vs. NMetM patients (OR=12.2). All data remained significant according to multivariate analyses.
However, we failed to calculate ORs for analogous triple combination comprising f allele carriers, because six study subjects with Ff+ff plus ≥20 years of smoking and plus obesity were all MetM patients. Further large-scale studies are necessary for such assessments.
Roles of vitamin D in melanoma require further studies. Melanoma cell culture and xenograft experiments in mice highlighted that vitamin D poses tumoral and metastasis suppression effects53-55. Virtually all actions of vitamin D occur through VDR activation. Thus, any modification of VDR activities induced by VDR polymorphisms can affect vitamin D functions13. Deletion of VDR results in increased susceptibility to tumor formation and reduces ability of keratinocytes to clear UVB-induced DNA mutations13,56. VDR can bind to thousands of VDREs on human genome and up- or down-regulate hundreds of genes. Of interest, recent evidence showed a crosstalk between VDR and immune factors57. VDR cistrome analyses suggested that altered expression of VDR in colon cancer changes actions of VDR, thus affecting patient outcome58. A recent study showed that VDR genetic traits can modulate VDR protein expression in excised human melanoma tissues, which might have implications for effects of vitamin D activity on melanoma cells59.
Thus, future research should focus on complex gene interactions and biological pathways related to vitamin D, VDR, smoking, excessive fat, and environmental factors with melanoma. Improved comprehension of biomolecular pathways will support further progress in melanoma management60.
Study limitations and strengths
Limitations of our study include limited number of melanomas and high CIs for some categorical variables. Nonetheless, several ORs were statistically significant. Analysis by data stratification for combined variables in some cases resulted in comparison of groups with less than 10subjects. Thus, future large-scale studies are necessary to better assess the role of such combined variables. We focused on white residents in northern Italy. Thus, our results cannot be generalized to populations with different genetic backgrounds. By contrast, a critical strength of our study is highly defined ethnic background of subjects. This variable bears significance in genetic studies. Variability in racial distribution and genetic melanoma susceptibility among (and across) different countries suggests that melanoma studies should be performed in restricted and wellcharacterized ethnic groups7. Another strength of our study is the detailed reported information, including combinations of genetic and lifestyle factors.
Conclusions
Treatment-resistant metastatic cancer is the most significant contributor to cancer mortality worldwide. Thus, better understanding of factors contributing to development of metastatic cancer may increase likelihood of future improvements in patient management. Our data highlighted that in terms of VDR gene alteration by SNPs, vitamin D homeostasis plays roles in cutaneous melanoma and MetM, and these functions are further enhanced by individual smoking habits and BMI. Thus, our findings support a geneenvironment contribution to development of malignant melanoma, suggesting the value of genetic screening, smoking cessation10, and excessive fat prevention51.
We first suggest gene-environment effects, including smoking duration and obesity, and VDR genetic polymorphisms with cutaneous malignant melanoma in general and specifically with MetM. Current data may contribute to development of a personalized/precision management for melanoma patients. Such management may include screening of VDR polymorphisms and detailed assessment of smoking habits and BMI. Further investigations are necessary to substantiate and extend our findings to examine different ethnic groups and to identify biological pathways related to vitamin D, smoking, and excessive fat, which influence skin cancers.
Acknowledgements
The authors would like to thank all melanoma patients and healthy controls who participated in the study. The authors are also grateful to Silvio Brusaferro, Dionisio Cauci, Maria Pia Iustulin, and Renato Picco for their help in enrollment of healthy subjects and technicians Patrizia Nacci and Luca Bazzichetto (University of Udine) for their help in experimental analyses. Lastly, the authors would like to express their gratitude to Blase Billack (St. John’s University, NY, USA) for critical reading of the manuscript.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.GLOBOCAN 2012. Estimated cancer incidence, mortality and prevalence worldwide in 2012. World Health Organization and International Agency for Research on Cancer (IARC). Available at: http://globocaniarcfr. [Last accessed May 19, 2017].
2.Erdmann F, Lortet-Tieulent J, Schüz J, Zeeb H, Greinert R, Breitbart EW, et al. International trends in the incidence of malignant melanoma 1953-2008-are recent generations at higher or lower risk? Int J Cancer. 2013; 132: 385-400.
3.Arnold M, Holterhues C, Hollestein LM, Coebergh JWW, Nijsten T, Pukkala E, et al. Trends in incidence and predictions of cutaneous melanoma across Europe up to 2015. J Eur Acad Dermatol Venereol. 2014; 28: 1170-8.
4.EUCAN (2912). Malignant melanoma of the skin. Available at: ecoiarcfr/eucan/Canceraspx?Cancer=20.2012: [Last accessed February 3, 2017]
5.AIRTUM. The Italian Association of Cancer Registries. I numeri del cancro in Italia-2016. Available at: http://registritumori.it/itacan. accessed May 19, 2017.
6.Registro Tumori Regione Friuli Venezia Giulia (FVG) 2010. Available at: www.cro.sanita.fvg.it [Last accessed May 19, 2017]
7.Cecconi L, Busolin A, Barbone F, Serraino D, Chiarugi A, Biggeri A, et al. Spatial analysis of incidence of cutaneous melanoma in the Friuli Venezia Giulia region in the period 1995-2005. Geospat Health. 2016; 11: 422
8.Bonin S, Albano A, di Meo N, Gatti A, Stinco G, Zanconati F, et al. Cutaneous melanoma frequencies and seasonal trend in 20 years of observation of a population characterised by excessive sun exposure. Radiol Oncol. 2015; 49: 379-85.
9.Gandini S, Montella M, Ayala F, Benedetto L, Rossi CR, Vecchiato A, et al. Sun exposure and melanoma prognostic factors. Oncol Lett. 2016; 11: 2706-14.
10.Newton-Bishop JA, Davies JR, Latheef F, Randerson-Moor J, Chan M, Gascoyne J, et al. 25-Hydroxyvitamin D2/D3levels and factors associated with systemic inflammation and melanoma survival in the Leeds melanoma Cohort. Int J Cancer. 2015; 136: 2890-9.
11.Lee SM, Pike JW. The vitamin D receptor functions as a transcription regulator in the absence of 1, 25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2016; 164: 265-70.
12.Gandini S, Gnagnarella P, Serrano D, Pasquali E, Raimondi S. Vitamin D receptor polymorphisms and cancer. Adv Exp Med Biol. 2014; 810: 69-105.
13.Bikle DD, Oda Y, Tu CL, Jiang Y. Novel mechanisms for thevitamin D receptor (VDR) in the skin and in skin cancer. J Steroid Biochem Mol Biol. 2015; 148: 47-51.
14.Orlow I, Reiner AS, Thomas NE, Roy P, Kanetsky PA, Luo L, et al. Vitamin D receptor polymorphisms and survival in patients with cutaneous melanoma: a population-based study. Carcinogenesis. 2016; 37: 30-8.
15.Santonocito C, Capizzi R, Concolino P, Lavieri MM, Paradisi A, Gentileschi S, et al. Association between cutaneous melanoma, Breslow thickness and vitamin D receptor BsmI polymorphism. Br J Dermatol. 2007; 156: 277-82.
16.Gapska P, Scott RJ, Serrano-Fernandez P, Mirecka A, Rassoud I, Górski B, et al. Vitamin D receptor variants and the malignant melanoma risk: a population-based study. Cancer Epidemiol. 2009; 33: 103-7.
17.Randerson-Moor JA, Taylor JC, Elliott F, Chang YM, Beswick S, Kukalizch K, et al. Vitamin D receptor gene polymorphisms, serum 25-hydroxyvitamin D levels, and melanoma: UK case-control comparisons and a meta-analysis of published VDR data. Eur J Cancer. 2009; 45: 3271-81.
18.Mandelcorn-Monson R, Marrett L, Kricker A, Armstrong BK, Orlow I, Goumas C, et al. Sun exposure, vitamin D receptor polymorphisms FokI and BsmI and risk of multiple primary melanoma. Cancer Epidemiol. 2011; 35: e105-10.
19.Orlow I, Roy P, Reiner AS, Yoo S, Patel H, Paine S, et al. Vitamin D receptor polymorphisms in patients with cutaneous melanoma. Int J Cancer. 2012; 130: 405-18.
20.Zhao XZ, Yang BH, Yu GH, Liu SZ, Yuan ZY. Polymorphisms in the vitamin D receptor (VDR) genes and skin cancer risk in European population: a meta-analysis. Arch Dermatol Res. 2014; 306: 545-53.
21.Lee YH, Song GG. Vitamin D receptor FokI, BsmI, TaqI, ApaI, and EcoRV polymorphisms and susceptibility to melanoma: a metaanalysis. J BUON. 2015; 20: 235-43.
22.Hou W, Wan XF, Fan JW. Variants Fok1 and Bsm1 on VDR are associated with the melanoma risk: evidence from the published epidemiological studies. BMC Genet. 2015; 16: 14 doi: 10.1186/s12863-015-0163-6..
23.Thompson CA, Zhang ZF, Arah OA. Competing risk bias to explain the inverse relationship between smoking and malignant melanoma. Eur J Epidemiol. 2013; 28: 557-67.
24.Li Z, Wang Z, Yu Y, Zhang H, Chen L. Smoking is inversely related to cutaneous malignant melanoma: results of a meta-analysis. Br J Dermatol. 2015; 173: 1540-3.
25.IARC. International Agency for Research on Cancer. Available at: http://monographs.iarc.fr/ENG/Classification/. accessed May 19, 2017.
26.Odenbro Å, Gillgren P, Bellocco R, Boffetta P, Håkansson N, Adami J. The risk for cutaneous malignant melanoma, melanoma in situ and intraocular malignant melanoma in relation to tobacco use and body mass index. Br J Dermatol. 2007; 156: 99-105.
27.Song FJ, Qureshi AA, Gao X, Li T, Han JL. Smoking and risk of skin cancer: a prospective analysis and a meta-analysis. Int J Epidemiol. 2012; 41: 1694-705.
28.DeLancey JO, Hannan LM, Gapstur SM, Thun MJ. Cigarette smoking and the risk of incident and fatal melanoma in a large prospective cohort study. Cancer Causes Control. 2011; 22: 937-42.
29.Veierod MB, Thelle DS, Laake P. Diet and risk of cutaneous malignant melanoma: a prospective study of 50, 757 Norwegian men and women. Int J Cancer. 1997; 71: 600-4.
30.De Hertog SAE, Wensveen CAH, Bastiaens MT, Kielich CJ, Berkhout MJP, Westendorp RGJ, et al. Relation between smoking and skin cancer. J Clin Oncol. 2001; 19: 231-8.
31.Grange F, Barbe C, Aubin F, Lipsker D, Granel-Brocard F, Velten M, et al. Clinical and sociodemographic characteristics associated with thick melanomas. A population-based, case-case study in France. Arch Dermatol. 2012; 148: 1370-6.
32.Zinkhan M, Stang A, Jöckel KH, Marr A, Bornfeld N, Schmidt-Pokrzywniak A. Having children, social characteristics, smoking and the risk of uveal melanoma: a case-control study. Ophthalmic Epidemiol. 2013; 20: 360-8.
33.Jiang AJ, Rambhatla PV, Eide MJ. Socioeconomic and lifestyle factors and melanoma: a systematic review. Br J Dermatol. 2015; 172: 885-915.
34.Grignol VP, Smith AD, Shlapak D, Zhang X, Del Campo SM, Carson WE. Increased visceral to subcutaneous fat ratio is associated with decreased overall survival in patients with metastatic melanoma receiving anti-angiogenic therapy. Surg Oncol. 2015; 24: 353-8.
35.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Bodymass index and incidence of cancer: a systematic review and metaanalysis of prospective observational studies. Lancet. 2008; 371: 569-78.
36.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009; 27: 6199-206.
37.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988; 124: 869-71.
38.Colombini A, Brayda-Bruno M, Ferino L, Lombardi G, Maione V, Banfi G, et al. Gender differences in the VDR-FokI polymorphism and conventional non-genetic risk factors in association with lumbar spine pathologies in an Italian case-control study. Int J Mol Sci. 2015; 16: 3722-39.
39.Colombini A, Brayda-Bruno M, Lombardi G, Croiset SJ, Ceriani C, Buligan C, et al. BsmI, ApaI and TaqI polymorphisms in the vitamin D receptor gene (VDR) and association with lumbar spine pathologies: An Italian case-control study. PLoS One. 2016; 11: e0155004
40.Colombini A, Brayda-Bruno M, Lombardi G, Croiset SJ, Vrech V, Maione V, et al. FokI polymorphism in the vitamin D receptor gene (VDR) and its association with lumbar spine pathologies in the Italian population: a case-control study. PLoS One. 2014; 9: e97027 doi: 10.1371/journal.pone.0097027..
41.Colombini A, Cauci S, Lombardi G, Lanteri P, Croiset S, Brayda-Bruno M, et al. Relationship between vitamin D receptor gene (VDR) polymorphisms, vitamin D status, osteoarthritis and intervertebral disc degeneration. J Steroid Biochem Mol Biol. 2013;138: 24-40.
42.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015; 372: 793-5.
43.Uitterlinden AG, Fang Y, Van Meurs JBJ, Pols HAP, Van Leeuwen JPM. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004; 338: 143-56.
44.Lee SM, Riley EM, Meyer MB, Benkusky NA, Plum LA, DeLuca HF, et al. 1,25-Dihydroxyvitamin D3controls a cohort of vitamin D receptor target genes in the proximal intestine that is enriched for Calcium-regulating components. J Biol Chem. 2015; 290: 18199-215.
45.Zeljic K, Kandolf-Sekulovic L, Supic G, Pejovic J, Novakovic M, Mijuskovic Z, et al. Melanoma risk is associated with vitamin D receptor gene polymorphisms. Melanoma Res. 2014; 24: 273-9.
46.Denzer N, Vogt T, Reichrath J. Vitamin D receptor (VDR) polymorphisms and skin cancer: a systematic review. Dermatoendocrinol. 2011; 3: 205-10.
47.Ambatipudi S, Cuenin C, Hernandez-Vargas H, Ghantous A, Le Calvez-Kelm F, Kaaks R, et al. Tobacco smoking-associated genome-wide DNA methylation changes in the EPIC study. Epigenomics. 2016; 8: 599-618.
48.Taroni M, Zagà V, Bartolomei P, Gattavecchia E, Pacifici R, Zuccaro P, et al. 210Pb and 210Po concentrations in Italian cigarettes and effective dose evaluation. Health Phys. 2014; 107: 195-9.
49.Winters TH, Di Franza JR. Radioactivity in cigarette smoking. N Engl J Med. 1982; 306: 364-5.
50.Essa S, Reichrath S, Mahlknecht U, Montenarh M, Vogt T, Reichrath J. Signature of VDR miRNAs and epigenetic modulation of vitamin D signaling in melanoma cell lines. Anticancer Res. 2012; 32: 383-9.
51.Arnold M, Leitzmann M, Freisling H, Bray F, Romieu I, Renehan A, et al. Obesity and cancer: an update of the global impact. Cancer Epidemiol. 2016; 41: 8-15.
52.Li X, Liang LM, Zhang MF, Song FJ, Nan HM, Wang LE, et al. Obesity-related genetic variants, human pigmentation, and risk of melanoma. Hum Genet. 2013; 132: 793-801.
53.Yudok K, Matsuno H, Kimura T. 1α, 25-dihydroxyvitamin D3inhibits in vitro invasiveness through the extracellular matrix and in vivo pulmonary metastasis of B16 mouse melanoma. J Lab Clin Med. 1999; 133: 120-8.
54.Evans SR, Houghton AM, Schumaker L, Brenner RV, Buras RR, Davoodi F, et al. Vitamin D receptor and growth inhibition by 1, 25-dihydroxyvitamin D3in human malignant melanoma cell lines. J Surg Res. 1996; 61: 127-33.
55.Eisman JA, Barkla DH, Tutton PJ. Suppression of in vivo growth of human cancer solid tumor xenografts by 1, 25-dihydroxyvitamin D3. Cancer Res. 1987; 47: 21-5.
56.Danielsson C, Törmä H, Vahlquist A, Carlberg C. Positive and negative interaction of 1, 25-dihydroxyvitamin D3and the retinoid CD437 in the induction of human melanoma cell apoptosis. Int J Cancer. 1999; 81: 467-70.
57.Singh PK, Van Den Berg PR, Long MD, Vreugdenhil A, Grieshober L, Ochs-Balcom HM, et al. Integration of VDR genome wide binding and GWAS genetic variation data reveals co-occurrence of VDR and NF-κB binding that is linked to immune phenotypes. BMC Genomics. 2017; 18: 132 doi: 10.1186/s12864-017-3481-4..
58.Long MD, Campbell MJ. Integrative genomic approaches to dissect clinically-significant relationships between the VDR cistrome and gene expression in primary colon cancer. J Steroid Biochem Mol Biol. 2016, doi: 10.1016/j.jsbmb.2016.12.013.
59.La Marra F, Stinco G, Buligan C, Chiriacò G, Serraino D, Di Loreto C, et al. Immunohistochemical evaluation of vitamin D receptor (VDR) expression in cutaneous melanoma tissues and four VDR gene polymorphisms. Cancer Biol Med. 2017; 14: 162-75.
60.Rescigno T, Micolucci L, Tecce MF, Capasso A. Bioactive nutrients and nutrigenomics in age-related diseases. Molecules. 2017; 22: 105 doi: 10.3390/molecules22010105..
Cite this article as: Cauci S, Maione V, Buligan C, Linussio M, Serraino D, Stinco G, et al. BsmI (rs1544410) and FokI (rs2228570) vitamin D receptor polymorphisms, smoking, and body mass index as risk factors of cutaneous malignant melanoma in northeast Italy. Cancer Biol Med. 2017; 14: 302-18. doi: 10.20892/j.issn.2095-3941.2017.0064
Sabina Cauci
E-mail: sabina.cauci@uniud.it
May 19, 2017; accepted June 30, 2017.
Available at www.cancerbiomed.org
Copyright © 2017 by Cancer Biology & Medicine
 Cancer Biology & Medicine2017年3期
Cancer Biology & Medicine2017年3期
- Cancer Biology & Medicine的其它文章
- Survival after pulmonary metastasectomy in colorectal cancer patients: does a history of resected liver metastases worsen the prognosis? A literature review
- Expression levels of β-catenin and galectin-3 in meningioma and their effect on brain invasion and recurrence: a tissue microarray study
- Potential predictive factors for pathologic complete response after the neoadjuvant treatment of rectal adenocarcinoma: a single center experience
- Inhibition of IKK-NFκB pathway sensitizes lung cancer cell lines to radiation
- Preclinical and clinical applications of specific molecular imaging for HER2-positive breast cancer
- Natural and artificial small RNAs: a promising avenue of nucleic acid therapeutics for cancer
