How to establish an expected animal model of post-traumatic osteoarthritis?
Peng-Fei Han,Cheng-Long Chen,Zhi Tian,Peng-Cui Li,Lei Wei,Zhi Lv, Xiao-Chun Wei,*
aDepartment of Orthopedic Surgery,Second People's Hospital of Changzhi City,Changzhi,Shanxi 046010,China
bDepartment of Orthopedic Surgery,Second Clinical Medical College,Shanxi Medical University,Taiyuan,Shanxi 030001,China
How to establish an expected animal model of post-traumatic osteoarthritis?
Peng-Fei Hana,b,1,Cheng-Long Chenb,1,Zhi Tianb,Peng-Cui Lib,Lei Weib,Zhi Lvb, Xiao-Chun Weib,*
aDepartment of Orthopedic Surgery,Second People's Hospital of Changzhi City,Changzhi,Shanxi 046010,China
bDepartment of Orthopedic Surgery,Second Clinical Medical College,Shanxi Medical University,Taiyuan,Shanxi 030001,China
A R T I C L E I N F O
Article history:
23 February 2017
Accepted 1 April 2017
Available online 7 June 2017
Animal model
Osteoarthritis
Anterior cruciate ligament
Medial menisci
Post-traumatic osteoarthritis
Background:Animal models of osteoarthritis(OA),including post-traumatic osteoarthritis and spontaneous osteoarthritis,have been established in many ways.In recent years,there have been many reports in various foreign academic journals,but animal models of post-traumatic osteoarthritis(distinct from spontaneous osteoarthritis)have rarely been established or summarized in these reports.Animal models of post-traumatic osteoarthritis show different characteristics depending on the animal species and modeling methods used,which is why we have written this article.
Objective:To summarize the research progress and research status of animal models of post-traumatic osteoarthritis.
Methods:A retrospective review of the animal model of post-traumatic osteoarthritis(OA)was conducted on the basis of reports retrieved from the PubMed database with the keywords for searching“animal model,post-traumatic osteoarthritis(PTOA)”from October 2006 to October 2016 and con fi ded English language.A total of 80 academic articles on the study of animal models of traumatic osteoarthritis were retrieved,and 34 of them were included in this literature review after reading the free fulltext of them.
Results:Different PTOA models based on different modeling methods and different animal species had their own characteristics.Different modeling methods should be selected according to different modeling animals.
Conclusions:Considering the project funds,experimental objectives and technical conditions,appropriate experimental animal and modeling method should be selected based on synthetic considerations to obtain an appropriate PTOA model and ideal experimental results.
©2017 Shanxi Medical Periodical Press.Publishing services by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Osteoarthritis(OA)is a common in fl ammatory joint disease that affects a growing portion of the elderly and has a strong socioeconomic impact.1Because the pathogenesis and etiological factorsofpost-traumaticosteoarthritisarenotclear,thelackofeffective clinical diagnosis and treatment measures for the recovery of such patients has caused dif fi culties;therefore,it is necessary and urgent to studyOA byestablishing a traumatic osteoarthritisanimal model.
Animal models of post-traumatic osteoarthritis can be established by selecting different animal species through a variety ofarti fi cial means to induce progressive cartilage injury,subchondral bone reconstruction,osteophyte formation and soft tissue in fl ammation around the joints and other pathological processes.In this review,we summarize the research methods and current research situationofanimalmodelsofpost-traumaticosteoarthritis, covering nearly ten years of foreign academic papers,to provide appropriate reference methods for modeling post-traumatic osteoarthritis animal models in future studies.
2.Data and methods
2.1.Source
First,the authors searched the PubMed database from October 2006 to October 2016 for animal models of post-traumatic osteoarthritis using the keyword search of“animal model,post-traumaticosteoarthritis(PTOA)”withanEnglishlanguage restriction.
2.2.Inclusion criteria
The eligible literature should describe the related experimental study work and summarize the establishment and use of animal models of post-traumatic osteoarthritis.
2.3.Exclusion criteria
The exclusion criteria were an unreasonable experimental design and experimental methods and poorly described or repetitive description of the establishment of animal models.
2.4.Quality control
According to the inclusion criteria and exclusion criteria,the fi rst and second authors read the references carefully to obtain quali fi ed literature.
2.5.Search results
A total of 80 academic papers studying animal models of posttraumatic osteoarthritis were retrieved,and 34 of them were included in this review after reading the free full-text articles.
3.Results
3.1.Extra-articular induction:tibial compression overload model (7/34)
In 2012,Christiansen et al2used C57BL/6N mice and an overload cycle of the tibial compression method using a tibial compression system that consisted of two custom-built loading platens to establish a post-traumatic animal model.The bottom platen held the knee fl exed,and the top platen positioned the foot with the ankle at approximately 30°.Then,the right leg of each mouse was subjected to a single dynamic axial compressive load,which caused a transient anterior subluxation of the tibia relative to the distal femur(Fig.1).In view of the normal activities of the joint stress environment of articular cartilage,this modeling method is of great signi fi cance.Increasing or decreasing joint stress arti fi cially could result in PTOA.Bone matrix damage is caused when stress arti ficially increases or decreases.Due to compensatory hypertrophy and degeneration of cartilage cells,cartilage degeneration of the entire animal cartilage joint occurs.
Satkunananthan et al,3-5used C57BL/6N mice to successfully replicate the PTOA model in their experiments.Killian et al6successfully established a model by subjecting Flemish Giant rabbits to a blunt force insult to the tibiofemoral joint with compression axial overload.Tochigi et al7successfully molded the elbow joint PTOA model using agricultural pigs.The elbow joints received an axial compression overload.Borrelli et al8changed the position of the overload.New Zealand White rabbits received compression on the medial condyle of a femur to successfully simulate the course of human PTOA.
3.2.Intra-articular surgery
3.2.1.Anterior cruciate ligament transection,ACLT model(4/34)
In 1973,Pond et al9performed anterior cruciate ligament transection surgery in one of the hind limb knees of 10 dogs,and the contralateral joints were used as controls.The animals were sacri fi ced at different times 1-26 weeks after surgery.Radiological and pathologic examinations showed that the humanoid PTOA model was successfully established(Fig.2).This modeling principle generally indicates that the tibia should be constrained from the anterior cruciate ligament to limit its excessive movement.After the anterior cruciate ligament of the animal model was removed, forward displacementof the tibial and hind limb knee joint rotation inward increased the fl exion and extension processes of the animal's hind limb knee,resulting in joint stability destruction, eventually causing PTOA.
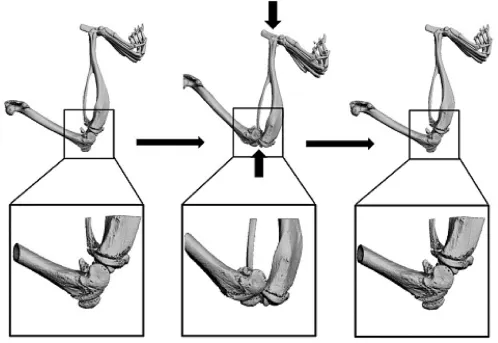
Fig.1.Tibial compression overload model.
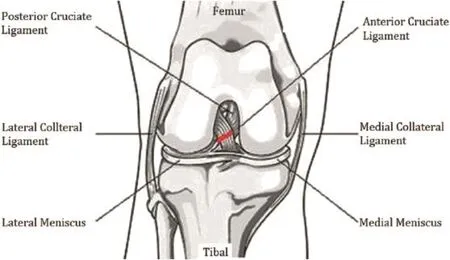
Fig.2.Anterior cruciate ligament transection(ACLT)model.
The canine ACLT model demonstrated that the animals recovered to a preoperative level 5 months after ACLT surgery by observing their ground reaction force(GRFs)and hind knee joint mechanics data.In 2005,Boyd et al10divided 13 cats into three groups and used the same ACTL method to mold the humanoid PTOA model to eliminate interference from the species variance in animal models.Then,they used Micro-CT to observe the long-term effects of bone around the joint(including the proximal tibia subchondral bone,femoral condyle and metaphyseal cancellous bone) and assess its effects in the pathogenesis of PTOA.10Similarly,in 2014,Nagai et al11using Japanese White Rabbits and the ACTL technique to establish the rabbit PTOA model.In 2016,it was reported that Dong et al12successfully molded the mouse PTOA model and M′evel et al1used New Zealand white rabbits to model the humanoid PTOA model.These authors obtained the expected experimental results.
3.2.2.Destabilization of the medial meniscus,DMM model(9/34)
In 1973,Moskowitz et al13divided 82 New Zealand whiterabbits into three groups,the rabbits'right hind limb knee was opened from the anterior medial side under aseptic conditions to expose the knee joint intermediate chamber,and then,the anterior half of the medial meniscus appendixes associated with 1/3 of the meniscus was removed.The humanoid PTOA model was established(Fig.3).By contrast,Arunakul et al14used 16 New Zealand white rabbits to mold the PTOA model for use in the total meniscectomy(TMM)surgery method,resulting in instability of the medial meniscus of the animal knee.Similarly,Panahifar et al15applied the Medial Meniscectomy(MMx)technique to mold the humanoid PTOA model for use in Sprague-Dawley rats.
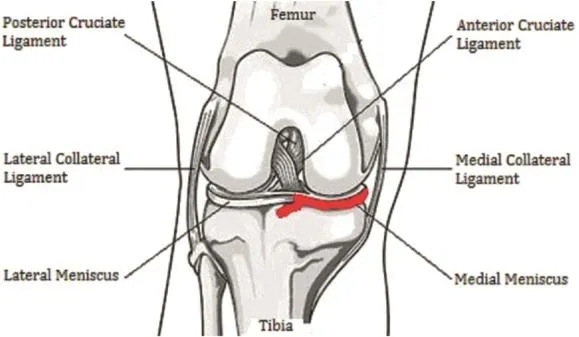
Fig.3.Destabilization of the medial meniscus(DMM)model.
The modeling process of the DMM model takes longer time than the ACTL model mentioned previously.16,17In their 2007 report, Glasson et al18used ADAMTS-4 and ADAMTS-5 gene knockout mice to compare the ACTL model and DMM model by evaluating the degree of cartilage damage with a histological scoring system.It was concluded that the ACTL model was able to mimic the severerhuman PTOA process,in which some ACTL animals show serious hind subchondral bone erosion and osteophyte formation in the tibial plateau.By contrast,the DMM model has less invasion;the tibial plateau and middle of the femoral condyle only demonstrate mild damage;therefore,Glasson et al18believe that DMM is the preferable operational method choice for mouse PTOA modeling. Usmani et al,16,19-21all adopted the above-mentioned viewpoint in the molding method in their experiments and successfully developed the murine PTOA model.
3.2.3.Hulth model/modi fi ed Hulth model(4/34)
According to the article“Experimental osteoarthritis in rabbits. Preliminary report”in 1970,Hulth and others removed the anterior cruciate ligament and medial collateral ligament,completely removed the medial meniscus using an experiment on rabbits,and successfully molded the humanoid PTOA model.The histological observation showed that the characteristics of PTOA,such as cartilage surface cracks and damage on the cartilage surface,were observed 3 months after Hulth's surgery(Fig.4).Hulth's surgery destroyed the main ligaments(including the anterior cruciate ligament and medial collateral ligament)that maintain the static stability of the knee joint of the animal.As a result,the normal force line of the knee changed from eversion of 10°tovarus,and the axial pressure of the joint changed,with the normal lateral tibial plateau moving to the medial side so that the weight-bearing surface of the joint decreased.In addition,the stress concentration increased the local articular cartilage compression,resulting in joint stability damage and aggravating articular surface wear to eventually cause PTOA.However,Moskowitz et al13argued that it is not suitable to observe biochemical metabolism changes of PTOA because the traditional Hulth model induces large trauma and more damage to the joint.It also has shorter modeling time and treatment intervening period,so the Hulth model PTOA is less commonly used in drug intervention in animal models.
Currently,improved Hulth models are available,such as ACLT+Partial meniscectomy(PM).The surgical approach is ACLT+medial meniscus resection.22-26In ACLT+Medial collateral ligament transaction(MCLT)+Medial meniscus resection(Mx),the surgical procedure aims to preserve the integrity of the posterior cruciate ligament,and the rest of procedures are the same as in the traditional Hulth model.27,28In cruciate ligament transection(CLT), the surgical approach is to only remove the anterior cruciate ligament;the rest of the static structure was not touched.29,30
3.2.4.Anterior cruciate ligament(ACL)auto graft anatomic reconstruction,ACL-R model(1/34)
In view of the above-mentioned various modeling methods,all of the methods focus on the joint instability pathway by destroying the joint stability structure and aggravating articular surface wear as well as using other surgical techniques to simulate the human PTOA process.However,we found that in the long term,the PTOA performance will still appear in some patients,even if their cruciate ligament has been reconstructed and they already have a relatively stable joint structure.To better understand and explore the pathogenesis of PTOA,Heard and others conducted an experiment on sheep using the autologous ligament graft autograft reconstruction method after anterior cruciate ligament surgery and eventually molded the humanoid PTOA model.31Increased matrix metalloproteinases as well as interleukin-like PTOA in fl ammatory markers could be observed in joint fl uid 2 weeks after surgery (Fig.5).
This model has eliminated the in fl uence of the instability of the joint structure on the incidence of PTOA,sowe can fully understand the in fl ammatory factors and early catabolic effects on the onset of PTOA,providing suf fi cient time to address the drug treatment window;therefore,the authors of this paper believe that this model is superior to the previous model,and we can regard this model as an idealized large animal model for the early diagnosis and treatment of PTOA(especially for studies of in fl ammatory factors and catabolic mechanisms).
3.2.5.Intra-articular fracture(IAF)model(3/34)
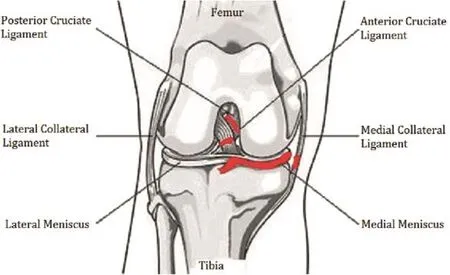
Fig.4.Hulth model and improved Hulth model.
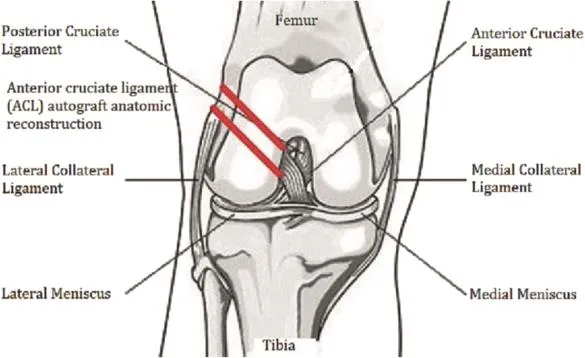
Fig.5.Anterior cruciate ligament autologous in situ reconstruction(ACL-R)model.
Goetz et al32used Yucatan miniature pigs to simulate the pathogenic course of PTOA using a special tripod fi xation,using immobilization after a tibial osteotomy of 1-2 mm of the pig knee (Fig.6).33Lewis et al34used C57BL/6 mice to mold a humanoid PTOA model using the arti fi cial compression axial overload method to cause an animal tibial plate fracture.Due to equipment limitations(such as a special tripod and compression table)and the corresponding internal fi xation materials,the authors do not recommend this method of establishing the models.
4.Discussion
In summary,after a retrospective review of the animal model of post-traumatic osteoarthritis in the PubMed database,it was determined that there are a variety of methods to model posttraumatic osteoarthritis(mainly in the knee)by using animal model,and the authors believe that the animal models of traumatic osteoarthritis show different characteristics,depending on the animal species and modeling methods used.In other words, different modeling methods should be selected according to the use of different model animals.The reported methods in the posttraumatic osteoarthritis animal model scan be found in Table 1.
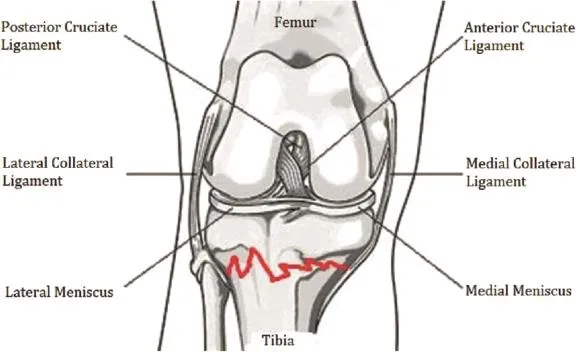
Fig.6.Intra-articular fracture(IAF)model.
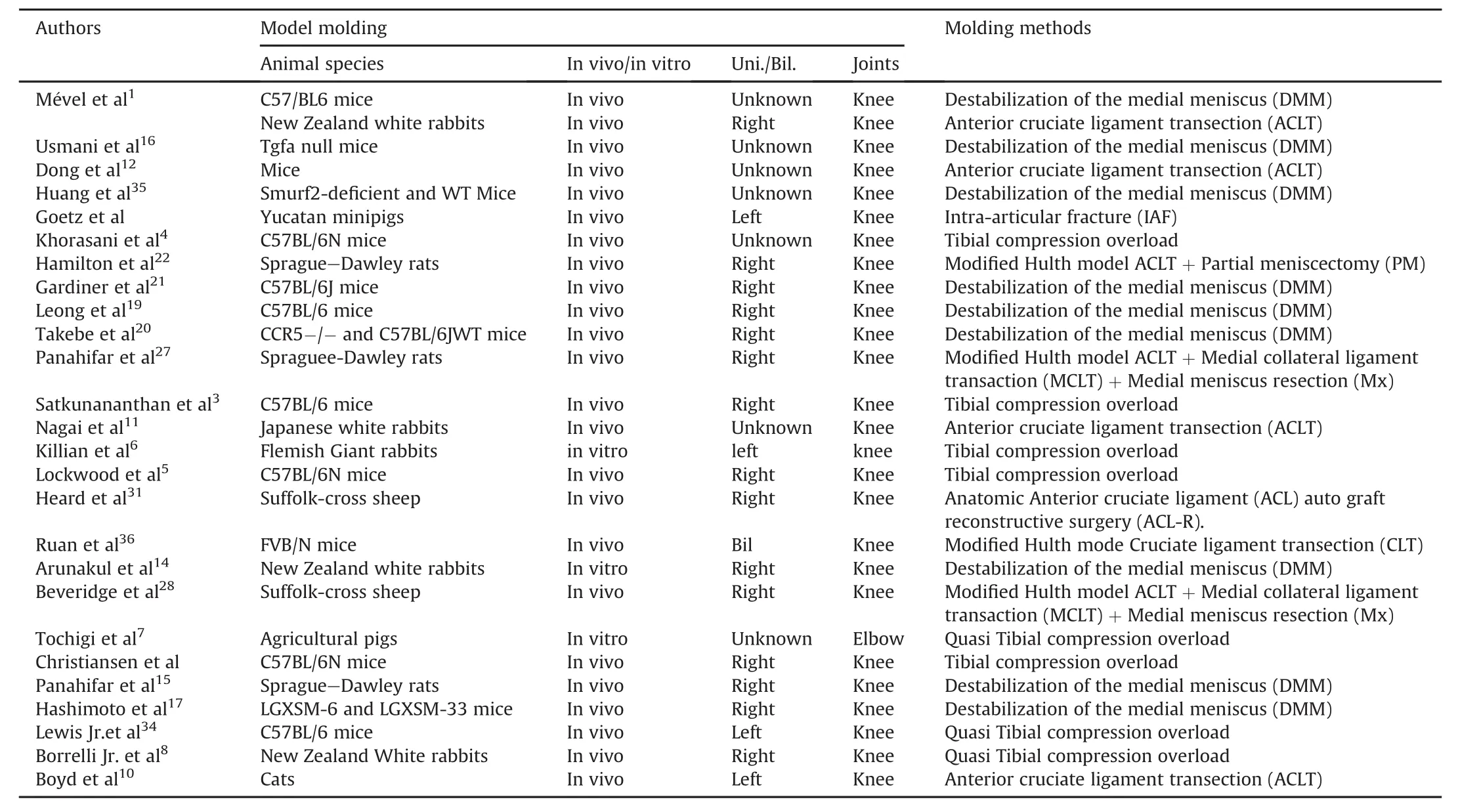
Table 1 Animal models of traumatic osteoarthritis in the literature.
As mentioned earlier,many papers have reported on molding animal models of osteoarthritis and developing spontaneous osteoarthritis animal models under natural conditions.The molding method is more mature and lacks deliberate human handling. The molding method can also lead to the use of a variety of animal species.Therefore,the aim of this paper was to summarize and analyze the methods of molding PTOA models by arti fi cial induction,namely,intra-articular surgical methods,such as anterior cruciate ligament transection(ACLT),the Destabilization of the medial meniscus(DMM)Model,the Hulth model/modi fi ed Hulth model,and so on.Because those methods(anterior cruciate ligament transection,destabilization of the medial meniscus etc.)are simple and easy to operate over a short period of time and the model is stable and reliable,it can be quickly applied in experimental research and widely used by scholars.However,we should also be aware that traumatic synovial in fl ammation caused by surgical trauma may interfere with articular cartilage and the biochemical metabolism of the synovial membrane.Therefore,this model is not suitable for the study of the biochemical metabolism of PTOA.However,non-surgical methods,such as the tibial compression overload model,are suitable for exploring PTOA biochemical metabolic research because it avoids surgerycaused by the traumatic synovitis effect.In addition,it is useful for studying post-traumatic articular cartilage components and changes in the expression of in fl ammatory mediators or other aspects.In particular,anterior cruciate ligament(ACL)auto graft anatomic reconstruction,the ACL-R model,is distinguished from all of the abovementioned modeling methods by focusing on joint destabilization, which by aggravating articular surface wear and using other surgical techniques,simulates human PTOA pathogenesis.This model has advantages for long-term study of the pathogenesis of PTOA after post-cruciate ligament reconstruction surgery and can be used as an idealized animal model for the early diagnosis and treatment of PTOA.
Animal models of traumatic osteoarthritis have different characteristics,depending on the animals and modeling methods used. There is no standard animal model for the PTOA modeling.37The authors suggest selecting appropriate experimental animals and modeling methods based on consideration of the project funds, experimental objectives and technical conditions to obtain the appropriate PTOA model and expected experimental results to provide guidance for clinical therapy.
Con fl icts of interest
All contributing authors declare no con fl icts of interest.
1.M′evel E,Merceron C,Vinatier C,et al.Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro anti IL-1βactivities before and after oral consumption.Sci Rep.2016;6:33527.
2.Christiansen BA,Anderson MJ,Lee CA,Williams JC,Yik JH,Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis.Osteoarthritis Cartilage.2012;20: 773-782.
3.SatkunananthanPB,AndersonMJ,DeJesusNM,HaudenschildDR, Ripplinger CM,Christiansen BA.In vivo fl uorescence re fl ectance imaging of protease activity in a mouse model of post-traumatic osteoarthritis.Osteoarthritis Cartilage.2014;22:1461-1469.
4.Khorasani MS,Diko S,Hsia AW,et al.Effect of alendronate on post-traumatic osteoarthritis induced by anterior cruciate ligament rupture in mice.Arthritis Res Ther.2015;17:30.
5.Lockwood KA,Chu BT,Anderson MJ,Haudenschild DR,Christiansen BA.Comparison of loading rate-dependent injury modes in a murine model of posttraumatic osteoarthritis.J Orthop Res.2014;32:79-88.
6.Killian ML,Haut RC,Haut Donahue TL.Acute cell viability and nitric oxide release in lateral menisci following closed-joint knee injury in a lapine model of post-traumatic osteoarthritis.BMC Musculoskelet Disord.2014;15:297.
7.Tochigi Y,Zhang P,Rudert MJ,et al.A novel impaction technique to create experimental articular fractures in large animal joints.Osteoarthritis Cartilage. 2013;21:200-208.
8.Borrelli Jr J,Zaegel MA,Martinez MD,Silva MJ.Diminished cartilage creep propertiesandincreasedtrabecularbonedensityfollowingasingle,sub-fracture impact of the rabbit femoral condyle.J Orthop Res.2010;28:1307-1314.
9.Pond MJ,Nuki G.Experimentally-induced osteoarthritis in the dog.Ann Rheum Dis.1973;32:387-388.
10.Boyd SK,Müller R,Leonard T,Herzog W.Long-term periarticular bone adaptation in a feline knee injury model for post-traumatic experimental osteoarthritis.Osteoarthritis Cartilage.2005;13:235-242.
11.Nagai T,Sato M,Kobayashi M,Yokoyama M,Tani Y,Mochida J.Bevacizumab, an anti-vascular endothelial growth factor antibody,inhibits osteoarthritis. Arthritis Res Ther.2014;16:427.
12.Dong Y,Liu H,Zhang X,et al.Inhibition of SDF-1α/CXCR4 signalling in subchondral bone attenuates post-traumatic osteoarthritis.Int J Mol Sci.2016;17: E943.
13.Moskowitz RW,Davis W,Sammarco J,et al.Experimentally induced degenerative joint lesions following partial meniscectomy in the rabbit.Arthritis Rheum.1973;16:397-405.
14.Arunakul M,Tochigi Y,Goetz JE,et al.Replication of chronic abnormal cartilage loading by medial meniscus destabilization for modeling osteoarthritis in the rabbit knee in vivo.J Orthop Res.2013;31:1555-1560.
15.Panahifar A,Maksymowych WP,Doschak MR.Potential mechanism of alendronate inhibition of osteophyte formation in the rat model of posttraumatic osteoarthritis:evaluation of elemental strontium as a molecular tracer of bone formation.Osteoarthritis Cartilage.2012;20:694-702.
16.Usmani SE,Ulici V,Pest MA,Hill TL,Welch ID,Beier F.Context-speci fi c protection of TGFαnull mice from osteoarthritis.Sci Rep.2016;6:30434.
17.Hashimoto S,Rai MF,Janiszak KL,Cheverud JM,Sandell LJ.Cartilage and bonechangesduringdevelopmentofpost-traumaticosteoarthritisin selected LGXSM recombinant inbred mice.Osteoarthritis Cartilage.2012;20: 562-571.
18.Glasson SS,Blanchet TJ,Morris EA.The surgical destabilization of the medial meniscus(DMM)model of osteoarthritis in the 129/SvEv mouse.Osteoarthritis Cartilage.2007;15:1061-1069.
19.Leong DJ,Choudhury M,Hanstein R,et al.Green tea polyphenol treatment is chondroprotective,anti-in fl ammatory and palliative in a mouse post-traumatic osteoarthritis model.Arthritis Res Ther.2014;16:508.
20.Takebe K,Rai MF,Schmidt EJ,Sandell LJ.The chemokine receptor CCR5 plays a role in post-traumatic cartilage loss in mice,but does not affect synovium and bone.Osteoarthritis Cartilage.2015;23:454-461.
21.Gardiner MD,Vincent TL,Driscoll C,et al.Transcriptional analysis of microdissected articular cartilage in post-traumatic murine osteoarthritis.Osteoarthritis Cartilage.2015;23:616-628.
22.Hamilton CB,Pest MA,Pitelka V,Ratneswaran A,Beier F,Chesworth BM. Weight-bearing asymmetry and vertical activity differences in a rat model of post-traumatickneeosteoarthritis.OsteoarthritisCartilage.2015;23: 1178-1185.
23.Appleton CT,McErlain DD,Pitelka V,et al.Forced mobilization accelerates pathogenesis:characterization of a preclinical surgical model of osteoarthritis. Arthritis Res Ther.2007;9:R13.
24.McErlain DD,Ulici V,Darling M,et al.An in vivo investigation of the initiation and progression of subchondral cysts in a rodent model of secondary osteoarthritis.Arthritis Res Ther.2012;14:R26.
25.Appleton CT,Pitelka V,Henry J,Beier F.Global analyses of gene expression in early experimental osteoarthritis.Arthritis Rheum.2007;56:1854-1868.
26.McErlain DD,Appleton CT,Litch fi eld RB,et al.Study of subchondral bone adaptations in a rodent surgical model of OA using in vivo micro-computed tomography.Osteoarthritis Cartilage.2008;16:458-469.
27.Panahifar A,Jaremko JL,Tessier AG,et al.Development and reliability of a multi-modality scoring system for evaluation of disease progression in preclinical models of osteoarthritis:celecoxib may possess disease-modifying properties.Osteoarthritis Cartilage.2014;22:1639-1650.
28.Beveridge JE,Heard BJ,Shrive NG,Frank CB.Tibiofemoral centroid velocity correlates more consistently with cartilage damage than does contact path length in two ovine models of sti fl e injury.J Orthop Res.2013;31:1745-1756.
29.Ruan MZ,Patel RM,Dawson BC,Jiang MM,Lee BH.Pain,motor and gait assessment of murine osteoarthritis in a cruciate ligament transection model. Osteoarthritis Cartilage.2013;21:1355-1364.
30.Ruan MZ,Dawson B,Jiang MM,Gannon F,Heggeness M,Lee BH.Quantitative imaging of murine osteoarthritic cartilage by phase-contrast micro-computed tomography.Arthritis Rheum.2013;65:388-396.
31.Heard BJ,Solbak NM,Achari Y,et al.Changes of early post-traumatic osteoarthritis in an ovine model of simulated ACL reconstruction are associated with transient acute post-injury synovial in fl ammation and tissue catabolism. Osteoarthritis Cartilage.2013;21:1942-1949.
32.Goetz JE,Fredericks D,Petersen E,et al.A clinically realistic large animal model of intra-articular fracture that progresses to post-traumatic osteoarthritis. Osteoarthritis Cartilage.2015;23:1797-1805.
33.Olson SA,Furman BD,Kraus VB,Huebner JL,Guilak F.Therapeutic opportunities to prevent post-traumatic arthritis:lessons from the natural history of arthritis after articular fracture.J Orthop Res.2015;33:1266-1277.
34.Lewis JS,Hembree WC,Furman BD,et al.Acute joint pathology and synovial in fl ammation is associated with increased intra-articular fracture severity in the mouse knee.Osteoarthritis Cartilage.2011;19:864-873.
35.Huang H,Veien ES,Zhang H,Ayers DC,Song J.Skeletal characterization of Smurf2-de fi cient mice and in vitro analysis of Smurf2-de fi cient chondrocytes. PLoS One.2016;11:1-18.
36.Ruan MZ,Cerullo V,Cela R,et al.Treatment of osteoarthritis using a helperdependent adenoviral vector retargeted to chondrocytes.Mol Ther Methods Clin Dev.2016;3:16008.
37.Kuyinu EL,Narayanan G,Nair LS,Laurencin CT.Animal models of osteoarthritis:classi fi cation,update,and measurement of outcomes.J Orthop Surg Res. 2016;11:19.
How to cite this article:Han P-F,Chen C-L,Tian Z,et al.How to establish an expected animal model of post-traumatic osteoarthritis?.Chin Nurs Res.2017;4:57-63.http://dx.doi.org/10.1016/ j.cnre.2017.06.002
11 January 2017
*Corresponding author.
E-mail address:weixiaochuan11@126.com(X.-C.Wei).
Peer review under responsibility of Shanxi Medical Periodical Press.
1Peng-Fei Han and Cheng-Long Chen were considered as co- fi rst authors.
in revised form
- Frontiers of Nursing的其它文章
- Transitional care interventions to reduce readmission in patients with chronic obstructive pulmonary disease:A meta-analysis of randomized controlled trials
- A bibliometric study of the Journal of School Health:1965-2014☆
- In fl uence of mobile education on joint function and quality of life in patients after total hip arthroplasty☆
- Development of a frailty scale for elderly people in China☆
- Chewing gum for postoperative ileus after colorectal surgery: A systematic review of overlapping meta-analyses
- Retraction notice to“Relationships between perceived social support and retention patients receiving methadone maintenance treatment in China mainland”[CNR 3/1(2016)11-15]

