Short-term observations of the regenerative potential of injured proximal sensory nerves crossed with distal motor nerves
Xiu-xiu Zhang, Yu-hui Kou, Xiao-feng Yin, Bao-guo Jiang, Pei-xun Zhang
Department of Orthopedics and Trauma, Peking University People’s Hospital, Beijing, China
Short-term observations of the regenerative potential of injured proximal sensory nerves crossed with distal motor nerves
Xiu-xiu Zhang, Yu-hui Kou, Xiao-feng Yin, Bao-guo Jiang, Pei-xun Zhang*
Department of Orthopedics and Trauma, Peking University People’s Hospital, Beijing, China
How to cite this article:Zhang XX, Kou YH, Yin XF, Jiang BG, Zhang PX (2017) Short-term observations of the regenerative potential of injured proximal sensory nerves crossed with distal motor nerves. Neural Regen Res 12(7):1172-1176.
Funding: This research was supported by a grant from the Ministry of Science and Technology 973 Project Planning of China, No. 2014CB542201; a grant from National High-Technology Research and Development Program of China (863 Program), No. SS2015AA020501; the National Natural Science Foundation of China, No. 31571235, 31571236, 31271284, 31171150; a grant from the Ministry of Education Innovation Team of China, No. IRT1201; the Educational Ministry New Century Excellent Talents Support Project of China, No. BMU20110270; a grant from the Ministry of Health of the Public Welfare Industry Special Scienti fi c Research of China, No. 201302007.
Graphical Abstract
orcid: 0000-0003-0929-6293 (Pei-xun Zhang)
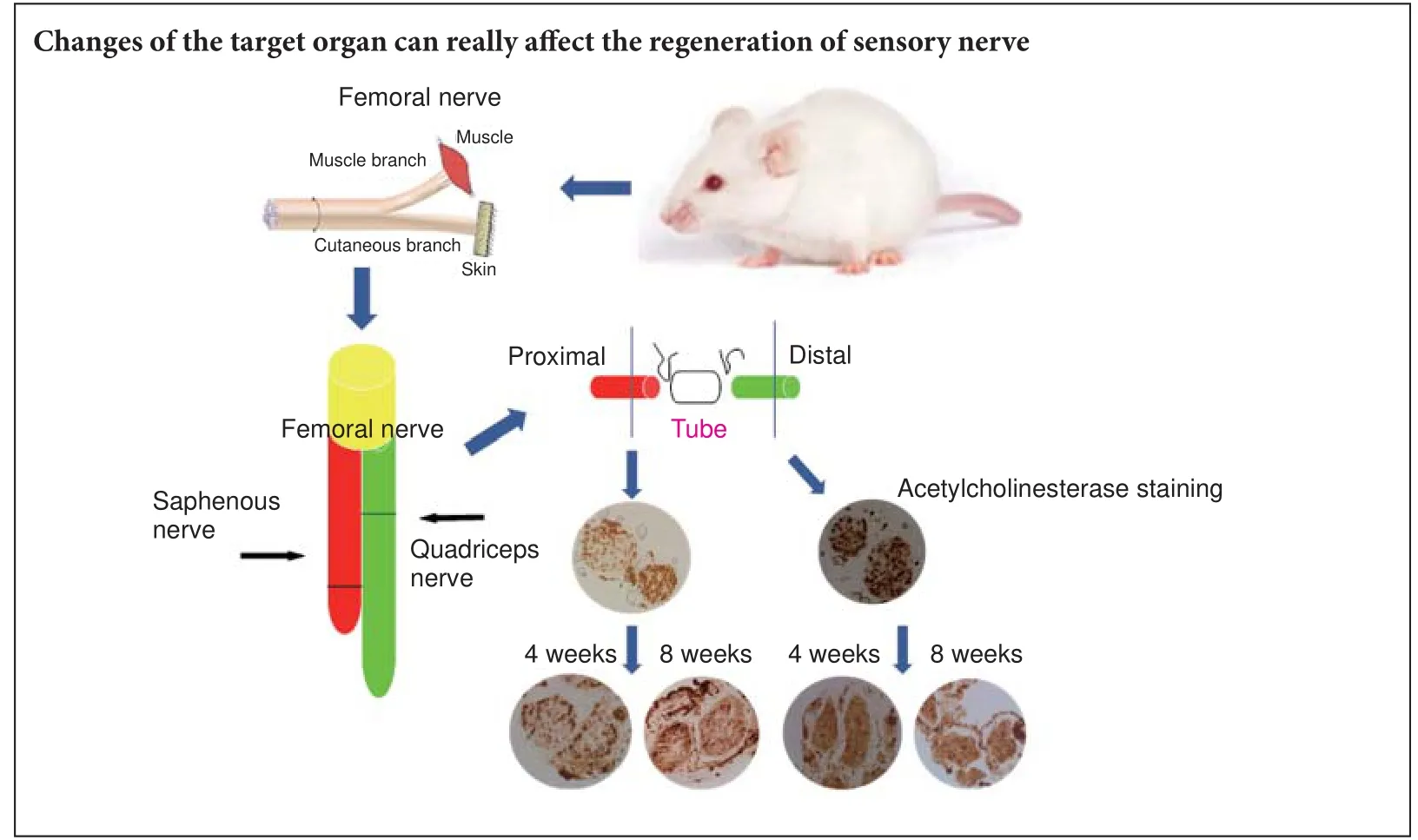
Motor nerves and sensory nerves conduct signals in di ff erent directions and function in di ff erent ways. In the surgical treatment of peripheral nerve injuries, the best prognosis is obtained by keeping the motor and sensory nerves separated and repairing the nerves using the suture method. However, the clinical consequences of connections between sensory and motor nerves currently remain unknown. In this study, we analyzed the anatomical structure of the rat femoral nerve, and observed the motor and sensory branches of the femoral nerve in the quadriceps femoris. Aer ligation of the nerves, the proximal end of the sensory nerve was connected with the distal end of the motor nerve, followed by observation of the changes in the newly-formed regenerated nerve fi bers. Acetylcholinesterase staining was used to distinguish between the myelinated and unmyelinated motor and sensory nerves. Denervated muscle and newly formed nerves were compared in terms of morphology, electrophysiology and histochemistry. At 8 weeks aer connection, no motor nerve fi bers were observed on either side of the nerve conduit and the number of nerve fi bers increased at the proximal end.e proportion of newly-formed motor and sensory fi bers was di ff erent on both sides of the conduit.e area occupied by autonomic nerves in the proximal regenerative nerve was limited, but no distinct myelin sheath was visible in the distal nerve.ese results con fi rm that sensory and motor nerves cannot be e ff ectively connected. Moreover, the change of target organ at the distal end a ff ects the type of nerves at the proximal end.
nerve regeneration; nerve remodeling; peripheral nerve; acetylcholinesterase staining; muscle denervation; neural anastomosis; nerve conduit; neural regeneration
Introduction
Materials and Methods
Animals
Eighteen 8-week-old female Sprague-Dawley rats weighing 200–250 g were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China (license No. SYXK (Jing) 2016-0009). All rats were housed in cages in speci fi c pathogen-free conditions under a 12-hour light/dark cycle with free access to food and water.e use of the animals was approved by the Ethics Committee and Experimental Animal Center of Peking University People’s Hospital (2013-59).
Establishment of nerve regeneration
All rats were intraperitoneally anesthetized using a 1% sodium pentobarbital solution and then randomly divided into three groups, with six rats in each group. In animals undergoing sensory-motor nerve crossing the femoral nerve on the right side was exposed and the saphenous and motor nerves were then transected at 5 mm and 3 mm from the neuromuscular junction, respectively.e proximal sensory nerve was then crossed at 2 mm from the distal quadriceps stump using a degradable chitin conduit with 9-0 nylon suture.e control group was used to monitor the status of the uninjured saphenous nerve.e e ff ects of sensory-motor nerve crossing were observed at 4 and 8 weeks postoperatively in di ff erent groups of animals.
Biological test
Postsurgical healing of the operative incisions, nutritional status and the extent of locomotor co-ordination of the right lower limb were monitored in all animals after surgery. The function of the lower limbs was measured by holding the tail of each rat while the rat attempted to hold onto a stick using the upper limbs, the ability of the rat to hold onto the stick using its lower limbs was then observed (Eberhardt et al. 2006).
Electrophysiological studies
Restoration ratio of quadriceps wet weight
After isolating the entire quadriceps muscle, the muscles were washed and dried using absorbent paper (Taizhou AoKe, Taizhou, China), and then weighed on an electronic scale (Yuyao JiMing, Zhejiang Province, China).e restoration ratio of muscle wet weight was determined using the formula: wet weightexperimentalside/wet weightcontrolside× 100%.
Hematoxylin-eosin staining
Acetylcholinesterase (AchE) staining
Paraformaldehyde- fi xed nerves located 5 mm proximal or 5 mm distal to the conduit were cut into 18-μm-thick sections. Nerve sections were stained to investigate the presence of AchE using the method previously described by Karnovsky and Roots (1964). The number of myelinated fibers was counted and the percentage of area of the unmyelinated nerves was determined and compared between sections. All images were evaluated using Image Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA) and PS CS3 (Adobe, San Jose, CA, USA) image-analysis software. All data obtained from rats undergoing sensory-motor nerve crossing werecompared with the equivalent data obtained using similar analyses of samples from control rats.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 statistical analysis soware (IBM, Armonk, NY, USA). All results are presented as the mean ± SE or the mean ± SD. Di ff erences between groups were tested for statistical signi fi cance using a pairedt-test. Di ff erences that exceeded the 95% con fi dence interval (P< 0.05) were considered statistically signi fi cant.
Results
Biological performance
All rats survived the surgical procedure. All the incisions healed, and no obvious differences in co-ordination were observed between the two lower limbs. When injured rats’grabbed a stick using their upper limbs, the right lower limbs appeared to fl ex, with a reduced ability to extend the knees.is phenomenon was more apparent among rats in the 8-week postoperative group (Figure 1).
Electrophysiological results
Histological and morphometric studies
The extent of atrophy of the quadriceps was significantly worse among rats in the 8-week postoperative group than among those in the 4-week postoperative group (29.7 ± 10.5%vs. 45.1 ± 13.1%,P≤ 0.05). Examinations of hematoxylin-eosin stained muscle sections from rats in the control group revealed the presence of a normal tissue architecture (Figure 3A), while similar examinations of muscle sections from rats in either of the two experimental groups revealed the presence of considerable nerve- fi ber atrophy (Figure 3B, C).
After AchE staining, the mean number of myelinated fibers in the saphenous nerve was 1,260.8 ± 107.5.e mean number of fibers in the same position among rats in the 4-week postoperative group was 1,609.8 ± 50.4, and 1,399.4 ± 102.1 among those in the 8-week postoperative group.e area of the autonomic nerves at the proximal end of the conduit in the general regenerative nerve was reduced compared with that of control mice (strong staining is indicated by the arrow in Figure 4).e percentage of the image area containing autonomic nerves in muscle sections from rats in the 4-week or 8-week postoperative groups was signi fi cantly lower than that of muscle sections obtained from rats in the control group (P= 0.005; Table 1). However, at the other end, dark staining was visible in the entire sections of the nerves, with an unclear myelin sheath (Figure 4). No motor fi bers were observed on two sides of the conduit in sections from either of the experimental groups.
Discussion
The optimal approach to the repair of injured peripheral nerves has been a subject of substantial research interest. In situations where nerve crossing has taken place during the repair process, the repaired fi bers of injured peripheral nerves could encounter motor, sensory, and/or autonomic nerves. If inappropriate matching of nerve stumps takes place during re-innervation, the subsequent nerve cannot become functional. Nevertheless, researchers have thus far been unable to explain the consequences of forming such nerves. Weiss (1935, 1945) attempted to sew the sensory nerve to denervated muscle. In the fi rst reported experiment of this kind, the aim was to determine whether or not a functional neuromuscular junction can form following such an approach. Although the results were negative, Weiss confirmed that the muscle fiber, which was connected to the sensory nerve, did become less atrophied. Hynes et al. (1997) also demonstrated that muscle atrophy was ameliorated following regeneration of the sensory nerve, and that the muscle fiber structure was also improved. However, as reported by the author, the conclusion was not convincing, because possible contributions of other regenerative pathways were not ruled out. Karpati et al. (1983), following extensive research, identified the presence of a newly formed sensory nerve and neuromuscular junction in previously denervated skeletal muscle, although these investigators still suggested that the regeneration was mediated by trophic support from motor nerves. Following these fi ndings, Bain et al. (2001) cut the tibial nerve and then sutured the transected distal tibial nerve stump to the common peroneal nerve, thus minimizing the possibility of regeneration owing to ectopic release of neurotrophic factors.e conclusion of these experiments was the same as those of previous investigations: the muscles were connected with sensory nerves, and the fi bers underwent great improvements in muscle structure and function, as demonstrated by improvements in muscle M-waves, twitch tension and tetanic tension.
At present, substantial evidence exists that the factors secreted by muscles or sensory nerves can provide trophic support to denervated muscle fi bers. For example, denervated muscle fi bers can be protected by the application of exogenous ciliary neurotrophic factor (Helgren et al., 1994). Furthermore, neurotrophin 3 and brain-derived neurotrophic factor have also been shown to promote the regeneration of skeletal muscle innervation (Karnovsky and Roots, 1964; Lohof et al., 1993; Kucera et al., 1995; Wang et al., 1995). Following injury, the newly-injured nerve will undergo changes in the neurotrophins it secretes, including increased levels of neurotrophin 4 and brain-derived neurotrophic factor (Funakoshi et al., 1993).

Figure 1 Weakness in extending the right knee.

Figure 2 Electrophysiological function in rats with and without sensory-motor nerve crosses.
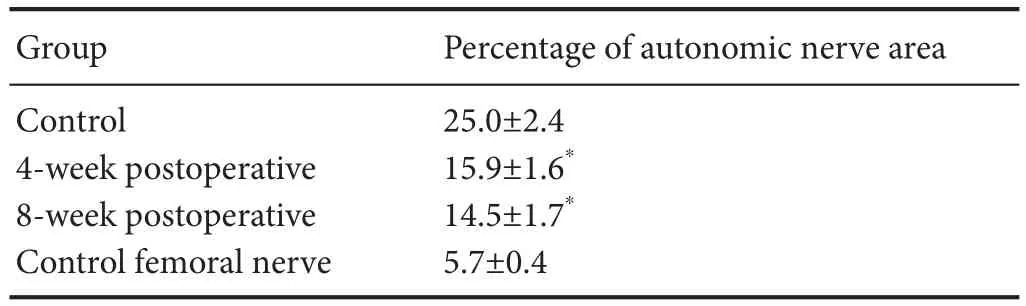
Table 1 Percentage of area occupied by autonomic nerves in the general saphenous nerve within 5 mm of the proximal end of the conduit in rat models of nerve regeneration, relative to controls

Figure 3 Morphometric changes in the quadriceps of rats that underwent sensory-motor nerve crosses (hematoxylin-eosin staining, × 100).
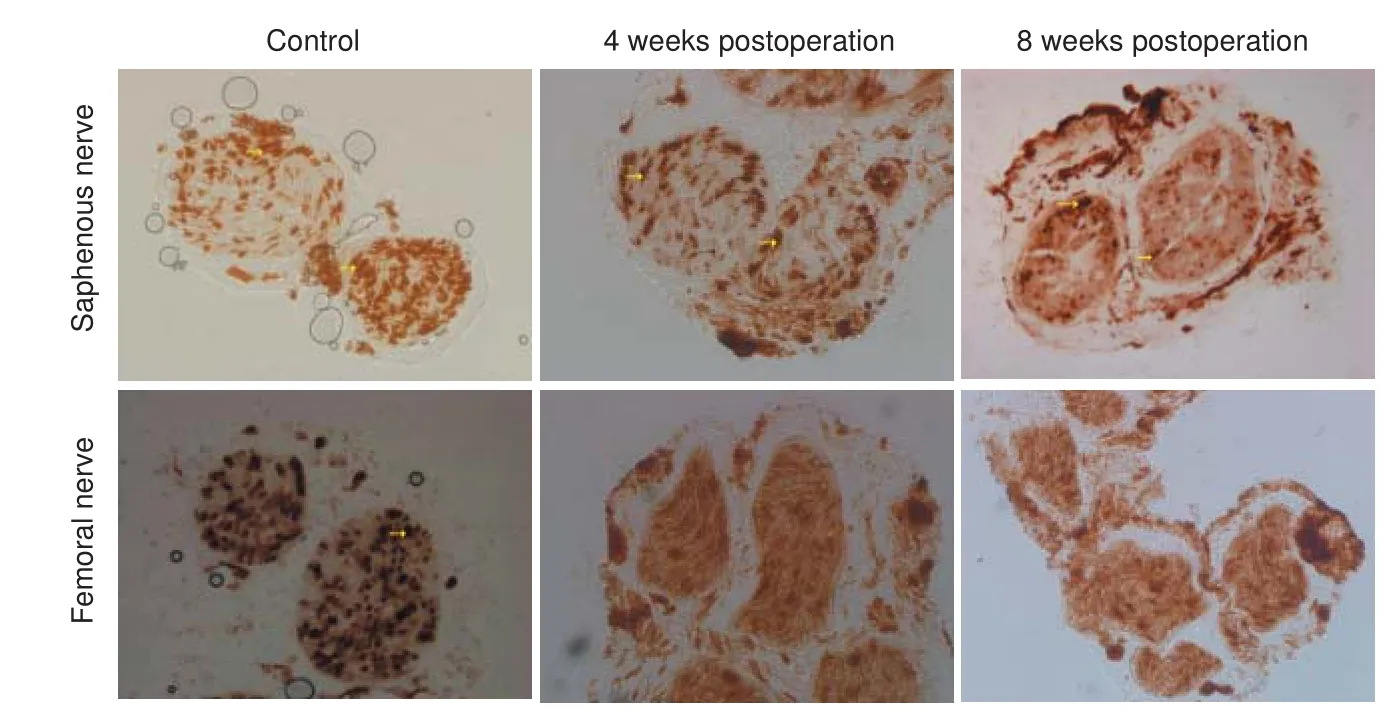
Figure 4 Morphological changes in the saphenous and femoral nerves following crossing of the proximal stump of the saphenous nerve to the distal stump of the quadriceps nerve (acetylcholinesterase staining, × 200).
The process of myelin sheath degradation after muscle denervation was originally described by Sunderland and Bradley (1950). Following myelin degradation, a basement membrane is generated by Schwann cells, which provides support for nerve repair and regeneration processes. Sensory nerves might have a role in the formation of the basement membrane (Bunge et al., 1982).e phenomenon of sensory-nerve-mediated trophic support promoting the regeneration of motor nerves has now been con fi rmed (Bunge et al., 1982); however, the mechanism of this e ff ect remains unknown. The aim of our experiments was to determine whether a change of target organ aer injury can a ff ect the newly-formed axon, and this information would then enable the mechanisms underlying sensory-nerve-mediated trophic support to be established through further research. At present, we know the rate of nerve regeneration is 1 mm/day in humans, and that nerve regeneration in rats is faster than in humans (Zhu, 2007). According to this speed of regeneration, the newly formed nerve can pass through the anastomosis at approximately 2 weeks aer nerve regeneration.e distance from the femoral nerve branches to themuscle in rats is approximately 1.5 cm; therefore, the results of the electrophysiological tests were expected to be positive, as reported elsewhere (Hynes et al., 1997); however, no nerve function was detected at either 4 or 8 weeks aer injury.is fi nding might indicate that insu ffi cient time was allowed for nerve regeneration to take place.
In this study, the number of newly-formed nerve fibers in the proximal stump increased at 4 weeks after surgery, and then decreased at 8 weeks. This decrease in the number of nerve fibers towards that of rats in the control group can be explained by an increase in the number of nerve fi bers sprouting from the proximal stump following injury. Furthermore, at 8 weeks aer surgery, the area of the autonomic nerves on the proximal side of the conduit in the regenerating nerve was signi fi cantly lower than that of the control group.e area containing the autonomic nerves was smaller in the motor nerve than in the sensory nerve, we suspect that the change of target organ resulted in alterations in the proportion of different nerve fi bers present in the newly formed sensory nerves. At the distal end of the conduit, strong staining was visible in whole sections of the nerves with a lack of an obvious myelin sheath.is apparent lack of myelination was very di ff erent from the proximal end, probably because aer motor-nerve collapse, the autonomic nerves are able to grow into the neural membrane of the original motor nerve having passed through the conduit because both nerves release the same neurotransmitter. Finally, the duration of the postsurgical observation period was too short for a recovery of nerve function to be observed, despite a recovery of nerve function being the expected fi nding of the electrophysiological experiments.
A notable weakness of this study is the lack of sham surgical procedures conducted in the control group. As a result of this, we cannot be certain whether or not all of the observed e ff ects are caused by the experimental procedures.
In this study, we attempted to change the target organ by crossing the proximal sensory nerve stump with the distal motor nerve stump, but e ff ective connection did not occur.e change of target organ appears to have altered the proportion of di ff erent nerve fi bers present in the newly-formed sensory nerves. These alterations indicate that changes in the target organ are able to affect sensory nerve regeneration. Aer the sensory branch is connected with the motor nerve, the proportion of di ff erent nerve fi bers present in the newly formed nerve is similar to that of the nerve that had the same source, meaning the same neurotransmitter or the same physiological role.
Author contributions:XXZ implemented the experiments, analyzed experimental data, draed the paper, and was responsible for statistical analysis. PXZ and BGJ conceived and designed the study. PXZ supervised the paper. PXZ and XFY were responsible for the funds. XFY and YHK provided technical or information support, and revised the study. All authors approved the fi nal version of the paper.
Con fl icts of interest:None declared.
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Bain JR, Veltri KL, Chamberlain D, Fahnestock M (2001) Improved functional recovery of denervated skeletal muscle after temporary sensory nerve innervation. Neuroscience 103:503-510.
Bunge MB, Williams AK, Wood PM (1982) Neuron-Schwann cell interaction in basal lamina formation. Dev Biol 92:449-460.
Eberhardt KA, Irintchev A, Al-Majed AA, Simova O, Brushart TM, Gordon T, Schachner M (2006) BDNF/TrkB signaling regulates HNK-1 carbohydrate expression in regenerating motor nerves and promotes functional recovery aer peripheral nerve repair. Exp Neurol 198:500-510.
Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H (1993) Di ff erential expression of mRNAs for neurotrophins and their receptors aer axotomy of the sciatic nerve. J Cell Biol 123:455-465.
Helgren ME, Squinto SP, Davis HL, Parry DJ, Boulton TG, Heck CS, Zhu Y, Yancopoulos GD, Lindsay RM, DiStefano PS (1994) Trophic e ff ect of ciliary neurotrophic factor on denervated skeletal muscle. Cell 76:493-504.
Karnovsky MJ, Roots L (1964) A “Direct-Coloring” thiocholine method for cholinesterases. J Histochem Cytochem 12:219-221.
Karpati G, Armani M, Carpenter S, Prescott S (1983) Reinnervation is followed by necrosis in previously denervated skeletal muscles of dystrophic hamsters. Exp Neurol 82:358-365.
Kucera J, Ernfors P, Walro J, Jaenisch R (1995) Reduction in the number of spinal motor neurons in neurotrophin-3-de fi cient mice. Neuroscience 69:321-330.
Lohof AM, Ip NY, Poo MM (1993) Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363:350-353.
Sunderland S, Bradley KC (1950) Denervation atrophy of the distal stump of a severed nerve. J Comp Neurol 93:401-409.
Wang T, Xie K, Lu B (1995) Neurotrophins promote maturation of developing neuromuscular synapses. J Neurosci 15:4796-4805.
Weiss P (1935) Experimental innervation of muscles by the central ends of a ff erent nerves (establishment of a one-neurone connection between receptor and e ff ector organ), with functional tests. J Comp Neurol 61:135-174.
Weiss P (1945) Sensory-motor nerve crossesin therat. J Neurophysiol 8:173-193.
Zhang P, Zhang L, Zhu L, Chen F, Zhou S, Tian T, Zhang Y, Jiang X, Li X, Zhang C, Xu L, Huang F (2015)e change tendency of PI3K/Akt pathway aer spinal cord injury. Am J Transl Res 7:2223-2232.
Zhu J (2007) E ff ects of immunosuppressant FK506 on nerve regeneration. In: Modern Peripheral Neurological Surgery (Gu LQ), pp236-243. Shanghai: Centry.
Copyedited by Sidaway P, Stow A, Yu J, Li CH, Qiu Y, Song LP, Zhao M
10.4103/1673-5374.211199
Accepted: 2017-05-26
*Correspondence to: Pei-xun Zhang, Ph.D., zhangpeixun@bjmu.edu.cn.
- 中国神经再生研究(英文版)的其它文章
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Recovery of sympathetic nerve function after lumbar sympathectomy is slower in the hind limbs than in the torso
- Aldehyde dehydrogenase 2 overexpression inhibits neuronal apoptosis after spinal cord ischemia/ reperfusion injury
- Long-term acupuncture treatment has a multitargeting regulation on multiple brain regions in rats with Alzheimer’s disease: a positron emission tomography study
- E ff ect of glycosides of Cistanche on the expression of mitochondrial precursor protein and keratin type II cytoskeletal 6A in a rat model of vascular dementia
- How does conserved dopamine neurotrophic factor protect against and rescue neurodegeneration of PC12 cells?

