Aldehyde dehydrogenase 2 overexpression inhibits neuronal apoptosis after spinal cord ischemia/ reperfusion injury
Xing-zhen Liu, Xin Sun, Kang-ping Shen, Wen-jie Jin, Zhi-yi Fu, Hai-rong Tao, Zhi-xing Xu
Shanghai Key Laboratory of Orthopedic Implants, Department of Orthopedic Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Aldehyde dehydrogenase 2 overexpression inhibits neuronal apoptosis after spinal cord ischemia/ reperfusion injury
Xing-zhen Liu#, Xin Sun#, Kang-ping Shen*, Wen-jie Jin*, Zhi-yi Fu, Hai-rong Tao, Zhi-xing Xu
Shanghai Key Laboratory of Orthopedic Implants, Department of Orthopedic Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
How to cite this article:Liu XZ, Sun X, Shen KP, Jin WJ, Fu ZY, Tao HR, Xu ZX (2017) Aldehyde dehydrogenase 2 overexpression inhibits neuronal apoptosis aer spinal cord ischemia/reperfusion injury. Neural Regen Res 12(7):1166-1171.
Graphical Abstract
#
orcid: 0000-0003-4355-3142 (Kang-ping Shen) 0000-0002-5521-7341 (Wen-jie Jin)
Aldehyde dehydrogenase 2 (ALDH2) is an important factor in inhibiting oxidative stress and has been shown to protect against renal ischemia/reperfusion injury.erefore, we hypothesized that ALDH2 could reduce spinal cord ischemia/reperfusion injury. Spinal cord ischemia/reperfusion injury was induced in rats using the modified Zivin’s method of clamping the abdominal aorta. After successful model establishment, the agonist group was administered a daily consumption of 2.5% alcohol. At 7 days post-surgery, the Basso, Beattie, and Bresnahan score signi fi cantly increased in the agonist group compared with the spinal cord ischemia/reperfusion injury group. ALDH2expression also signi fi cantly increased and the number of apoptotic cells signi fi cantly decreased in the agonist group than in the spinal cord ischemia/reperfusion injury group. Correlation analysis revealed that ALDH2expression negatively correlated with the percentage of TUNEL-positive cells (r= −0.485,P< 0.01). In summary, increased ALDH2expression protected the rat spinal cord against ischemia/ reperfusion injury by inhibiting apoptosis.
nerve regeneration; spinal cord ischemia/reperfusion injury; aldehyde dehydrogenase 2; alcohol; apoptosis; oxidative stress; terminal deoxynucleotidyl transferase dUTP nick-end labeling; neural regeneration
Introduction
Decompression of a previously severely compressed region of the spinal cord could induce spinal cord ischemia/reperfusion injury (SCII), which could lead to further irreversible postoperative complications, such as paraplegia or paraparesis (Taher et al., 2013; Zhu et al., 2013; Yang et al., 2015). SCII includes two phases: 1) immediate spinal cord injury related to acute ischemia; and 2) delayed spinal cord injury involving ischemic cellular death and reperfusion injury.
Although the molecular mechanisms of ischemia/reperfusion injury (IRI) pathogenesis remain poorly understood, evidence suggests that oxidative stress caused by excessive production of reactive oxygen species during the IRI process is a critical factor in direct and subsequent cellular damage (Noiri et al., 2001; Walker et al., 2001; Colón and Miranda, 2016). Lipid peroxidation, which is an important source of oxidative stress, is an autocatalytic mechanism resulting in oxidative destruction of cellular membranes associated with production of toxic, reactive, aldehydic metabolites and cell death (Niki et al., 2005). Reactive aldehydes, suchas 4-hydroxy-2-nonenal and malondialdehyde, the major end products of lipid peroxidation, are highly toxic and react with proteins to form various adducts, leading to dysfunctional proteins and subsequent cellular injury (Renner et al., 2005; Conklin et al., 2006; Marchitti et al., 2007). Previous studies of cardiac and renal IRI have suggested that preconditions with the e ff ective activator aldehyde dehydrogenase 2 (ALDH2) or physiological levels of ethanol provide protection for corresponding organs, which consistently correlates with the phosphorylation status of ALDH2, a key metabolic enzyme in the oxidation and detoxi fi cation of reactive aldehydes in a range of organs and cell types, to inhibit oxidative stress (Chen et al., 2008; Yuan et al., 2011). ALDH2is a mitochondrial enzyme and belongs to the ALDH gene family. ALDH2not only catalyzes oxidation of acetaldehyde to acetic acid in ethanol metabolism, but also acts as a key metabolic enzyme in the detoxi fi cation of other reactive aldehydes such as hydroxynonenal (Vasiliou et al., 2005). However, to the best of our knowledge, it is unknown whether ALDH2has neuroprotective effects by reducing apoptosis under conditions of SCII.e objective of our study was to investigate the early e ff ect of ALDH2on apoptosis through alcohol administration in rats subjected to SCII.
Materials and Methods
Animals
Thirty adult, male, Sprague-Dawley rats, aged 8–12 weeks and weighing 250–270 g, were obtained from the Animal Laboratory of Second Military Medical University of China (license number: SCXK (Hu) 2013-0006).e study protocol was approved by the Animal Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, China. The experimental procedure followed the the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986), and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association for Veterinary Editors (IAVE).
The rats were randomly divided into sham operation group (sham group,n= 10), SCII group (I/R group,n= 10), and SCII with alcohol group (agonist group,n= 10). Rats in the sham group underwent surgery without injury. Rats in the I/R and agonist groups su ff ered from SCII using Zivin’s method (Zivin et al., 1980). Aer successful model establishment, the agonist group received daily consumption of 2.5% alcohol (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China); the other groups were fed water (30 mL per day) for 7 days post-surgery.
SCII modeling and alcohol intervention
The rat model of SCII was performed employing the previously described Zivin’s method (Zivin et al., 1980). All rats were prohibited from drinking during the morning of the surgery. Rats were deeply anesthetized with 3% sodium pentobarbital (1 mL/kg, intraperitoneal injection) and placed in a supine position. Aer making a 4–5-cm medial incision, the abdominal aorta was exposed at the level of the lerenal artery. A total of 400 U heparin was injected 4 minutes before aortic occlusion, and spinal cord ischemia was induced by clamping the aorta with a bulldog clamp just below the lerenal artery. Aer occlusion, femoral artery pulsation ceased, and blood fl ow was obstructed for 40 minutes.e bulldog clamp was removed, and the abdominal wall was closed. Successful rat model of spinal cord ischemia criteria: cessation of abdominal aorta and doublehindlimb skin cyanosis. Signs of recanalization blood fl ow: once the arterial clamp was loosened, abdominal aorta beating recurred and color in the skin of both hindlimbs was bright red.

Figure 2 Aldehyde dehydrogenase 2 overexpression a ff ected histomorphology in rats with spinal cord ischemia/reperfusion injury (hematoxylin-eosin staining, ×200).
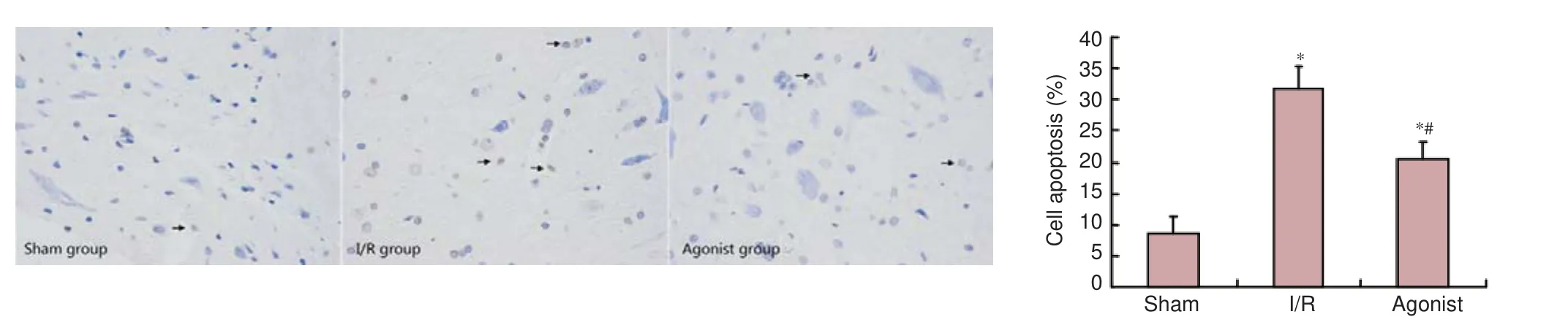
Figure 4 ALDH2overexpression a ff ected apoptosis in rats with spinal cord ischemia/reperfusion injury (TUNEL assay, × 400).
Motor function assessment
Hind limb motor function assessment was performed at 7 days post-surgery using the Basso, Beattie, and Bresnahan (BBB) motor rating scale (Basso et al., 1995).is BBB scale was based on motor ability following SCII in a rat model. Brie fl y, the BBB scale was a 22-point scale from 0 to 21.e 0-point indicated no observable hind limb movement, and 21 points indicated consistent and coordinated gait with parallel paw placement of the limbs and consistent trunk stability. Locomotion was scored by two independent observers blind to experimental design.
Sacri fi ce time
All rats were sacrificed at 7 days post-surgery, and the spinal cords (L2–5) were immediately removed to identify medullospinal pathologic changes, examine ALDH2 protein expression, and observe cell apoptosis, respectively, by hematoxylin-eosin staining, western blot assay, and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay.
Hematoxylin-eosin staining
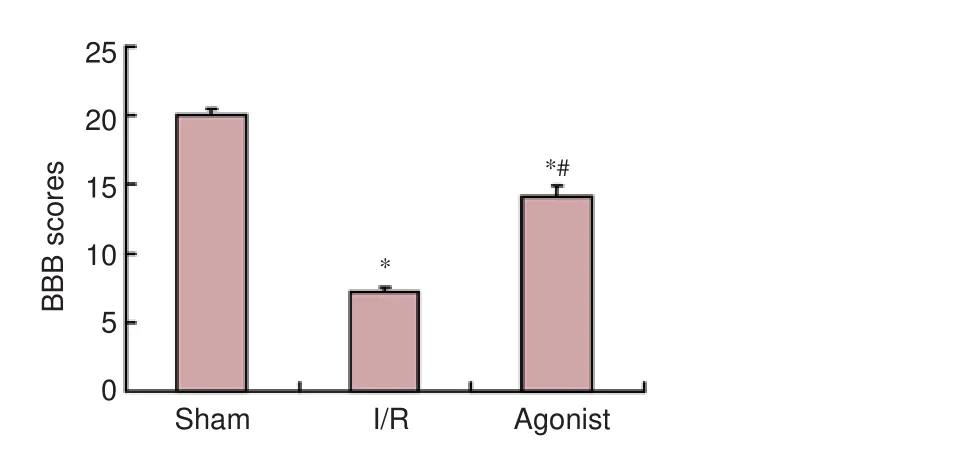
Figure 1 BBB scores in rats at 7 days aer surgery.
Western blot assay
The second of spinal cord (L2–5) sample was centrifuged at 1,500 r/min for 30 minutes, aer which the supernatant was collected. Determination of protein concentration was performed using the bicinchoninic acid protein assay kit. Aer transferring 0,15, 30, 60, 120, 180, 240, and 300 μL of bovine serum albumin (500 μg/mL) into each 2-mL centrifuge tube, 1× PBS was added to each tube to reach a volume of 300 μL. The final concentrations of bovine serum albumin in each centrifuge tube were 0, 25, 50, 100, 200, 300, 400, and 500 μg/mL. Working reagent was prepared by mixing 50 parts bicinchoninic acid reagent A with 1 part bicinchoninic acid reagent B (50:1, reagent A:B).e protein extract (20 μL) was placed in an appropriately labeled test tube, and diluted with PBS 30 times. An additional 200 μL of working reagent was added to each tube and mixed well.e tubes were covered and incubated at 37°C for 30 minutes. The spectrophotometer measured at 550 nm and was set to zero using a cuvette fi lled only with 1 × PBS. Sample absorbance was then measured within 10 minutes.e sample was electrophoresed in a 5% stacking gel with 80 V constant voltage for 20 minutes, followed by 110 V constant voltage for 3.5 hours. A total of 20 μL was resolved on an electrophoresis gel and then transferred onto polyvinylidene difluoride membranes. Primary antibody specific for ALDH2(goat monoclonal antibody) (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was diluted in Tris-buffered saline and Tween 20 (TBST), added to the membrane, and incubated for 60 minutes at room temperature. The membrane was then washed in TBST and incubated in secondary antibody (rabbit anti-goat horseradish peroxidase-labeled polyclonal antibody, 1:10,000; Santa Cruz Biotechnology) for 2 hours at room temperature, followed by TBST washing (3 times) and TBS washing (10 minutes). Protein bands were visualized using enhanced chemiluminescence reagent (Beyotime, Haimen, China), followed by exposure and were photographed to save the experimental results.e ratio of optical density values between ALDH2andGAPDH bands, which was an indicator of ALDH2protein expression, was measured using the Gel-Pro analyzer 4.5 (Media Cybernetics, Sarasota, FL, USA).
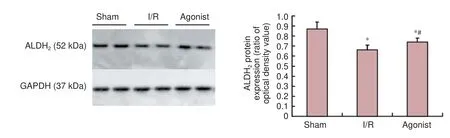
Figure 3 Protein expression in the spinal cord of ALDH2in the sham, I/R, and agonist groups (western blot assay).
TUNEL assay
Statistical analysis
Statistical analysis was performed using SPSS 19.0 soware (IBM, Armonk, IL, USA) and all data are expressed as the mean ± SD. Intergroup differences were analyzed by oneway analysis of variance and Student-Newman-Keuls test. Correlation analysis was examined by Pearson correlation coe ffi cient. Signi fi cance was set toP< 0.05.
Results
ALDH2overexpression a ff ected behavior in SCII rats
Rats in the sham group did not show obvious symptoms of neurological impairments. BBB scores in the I/R (7.20 ± 0.40) and agonist groups (14.10 ± 0.80) significantly decreased compared with the sham group (20.00 ± 0.50) (P< 0.05), and BBB scores signi fi cantly improved in the agonist group compared with the I/R group (P< 0.05; Figure 1).
ALDH2overexpression a ff ected histological changes in SCII rats
Hematoxylin-eosin staining results demonstrated well-maintained spinal cord tissues in the sham group, and neurons were morphologically normal with clear karyosomes, uniformly stained cytoplasm, and few vacuolar changes. In the I/R group, the number of normal neurons signi fi cantly decreased. Neurons were swollen and pyknotic, and the cytoplasm was stained dark with a great deal of vacuolization. In the agonist group, these pathological changes were markedly reduced compared with the I/R group, and the number of normal neurons was signi fi cantly greater than in the I/R group. Most neurons were multipolar with clear karyosomes, with few vacuolar changes (Figure 2).
ALDH2protein expression
ALDH2protein expression was signi fi cantly improved in the agonist group (0.74 ± 0.04) compared with the I/R group (0.66 ± 0.05) (P< 0.05; Figure 3).
ALDH2overexpression a ff ected apoptosis in SCII rats
TUNEL staining results demonstrated few TUNEL-positive cells in the sham group (8.47 ± 2.80).e percentage of TUNEL-positive cells was signi fi cantly higher in the I/R (31.69 ± 3.66) and agonist groups (20.45 ± 2.72) than in the sham group (P< 0.05). However, the percentage of TUNEL-positive cells was markedly decreased in the agonist group compared with the I/R group (P< 0.05; Figure 4).
Correlation between ALDH2expression and apoptotic rate
The Pearson correlation coefficient between ALDH2expression (X) and apoptotic rate (Y) was −0.485 in the three groups. Thus, ALDH2expression and apoptosis rate were negatively correlated (P< 0.01).
Discussion
Although adequate surgical decompression is the preferred treatment for chronic severe compression of the spinal cord and results in favorable surgical outcomes, SCII patients experience unpredictable and disastrous postoperative complications (Taher et al., 2013; Zhu et al., 2013; Yang et al., 2015). Because of the serious consequences of SCII, groups around the world have focused on how to effectively alleviate SCII.e present results demonstrated that moderate alcohol administration following SCII induction provides early protection. Aer establishment of a rat model of SCII, alcohol-treated rats displayed signi fi cantly preserved neurological function characterized by high BBB scores and less histological damage and apoptosis. Results further demonstrated that the protective e ff ect was associated with ALDH2. ALDH2is a crucial enzyme involved in protecting the IRI by inhibiting oxidative stress. ALDH2is one of 19 members of the ALDH gene family and is localized within the mitochondria, a major site for reactive oxygen species and reactive aldehyde generation (Vasiliou et al., 2005). ALDH2not only catalyzes oxidation of acetaldehyde to acetic acid in ethanol metabolism, but also acts as a key metabolic enzyme involved in the detoxi fi cation of other reactive aldehydes such as hydroxynonenal (Vasiliou et al., 2005; Chen et al., 2008; Ma et al., 2011; Yuan et al., 2011; Sun et al., 2014). Increasing evidence indicates that ALDH2plays a protective role in IRI of the heart, kidney, and brain. He et al. (2012) confirmed that the cardioprotective effects of alpha lipoic acid on IRI took place through a mechanism involving ALDH2activation. Ji et al. (2016) reported that ALDH2inhibited excessive mitophagy and increased cardiomyocyte following IRI by reducing 4-hydroxy-2-nonenal and reactive oxygen species levels. Yuan et al. (2011) and Zhong et al. (2016) reported that ALDH2provides protection for kidneys against IRI by enhancing antioxidant capacity and preventing lipid peroxidation. Fu et al. (2014) believed ALDH2activation could reduce cerebral IRI by decreasing accumulation of reactive aldehydes concomitantly with improvements in brain injury and neurological function. Results from the study clearly showed that increased ALDH2expression alleviated SCII in rats, which was consistent with the above analysis in other organs. In our study, the agonist group rats, which expressed increased levels of ALDH2, also exhibited significantly improved neurological functions as assessed by BBB scores compared with the I/R group at 7 days post-surgery. Additionally, the pathological changes were markedly reduced,and the number of normal neurons was signi fi cantly greater in the agonist group compared with the I/R group. Most neurons were multipolar with clear karyosomes, and there were few vacuolar changes.
Although the underlying mechanisms involved in ALDH2protection against IRI, previous studies have shown detoxication of reactive aldehydes, reduced reactive oxygen species generation, and protection of mitochondrial function terminally inhibiting endoplasmic reticulum stress, apoptosis, and autophagy (Ma et al., 2011; Pang et al., 2015).e present study focused on the relationship between ALDH2and apoptosis during SCII.
Inhibition of apoptosis could reduce the extent of injury and preserve neurological function (Lee et al., 2003; Li et al., 2016), which is closely associated with reactive oxygen species accumulation during IRI (Ebert et al., 2014).e anti-apoptotic enzyme ALDH2has been shown to be involved in cell apoptosis induced by oxidative stress (Zhang et al., 2011; Fan et al., 2013). Ebert et al. (2014) reported that defective regulation of ALDH2-dependent signaling events induces cardiomyocyte apoptosis resulting from increased 4-hydroxy-2-nonenal and reactive oxygen species. When ALDH2expression is stimulated, levels of 4-hydroxy-2-nonenal, reactive oxygen species, and apoptosis significantly decrease (Pang et al., 2015). Other studies also con fi rmed that increased ALDH2expression induced by alcohol or the e ff ective activator Alda-1 reduces cardiomyocyte apoptosis (Chen et al., 2008; Ge et al., 2012; Gao et al., 2015). Zhong et al. (2016) reported that increased ALDH2expression reduces renal cell apoptosis during IRI. Following liver injury, decreased ALDH2expression also leads to apoptosis (Zhong et al., 2016). However, it remained to be shown whether ALDH2exhibits neuroprotective e ff ects by reducing apoptosis in SCII. Results from the present study clearly showed that increased ALDH2expression stimulated by alcohol resulted in decreased SCII-induced apoptosis.
Although the molecular mechanisms involved in ALDH2and apoptosis remain poorly understood, increased ALDH2expression in fl uences apoptosis, which might be associated with subsequent activation of heat shock protein 70, phosphorylation of c-Jun N-terminal kinase, and inhibition of p53 (Li et al., 2004; Harada, et al., 2005; Sun et al., 2014; Zhong et al., 2016). Further studies are needed to determine the relationship between these molecules.
Our study has several limitations that deserve special attention. First, it is necessary to improve accuracy in this experiment by increasing the number of animal models. Second, to determine long-term neurological protection by ALDH2through alcohol administration, future studies should increase the observation interval time aer establishing the rat model of SCII.ird, this study preliminarily discussed the relationship between ALDH2and SCII, although further studies are needed to determine the involved mechanisms and contributions of ALDH2to SCII.
In summary, the neurological e ff ects of alcohol on SCII took place through a mechanism involving, at least in part, ALDH2activation, which effectively inhibited apoptosis. Results from this study could provide a novel therapeutic target for treating spinal cord injury induced by ischemia/ reperfusion.
Author contributions:XZL and KPS designed this study. XS, WJJ, HRT and ZXX performed experiments and analyzed experimental data. XS, XZL, WJJ, ZYF, HRT and ZXX were responsible for writing this paper. All authors approved the fi nal version of the paper.
Con fl icts of interest:None declared.
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open fi eld testing in rats. J Neurotrauma 12:1-21.
Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D (2008) Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science 321:1493-1495.
Colón JM, Miranda JD (2016) Tamoxifen: an FDA approved drug with neuroprotective e ff ects for spinal cord injury recovery. Neural Regen Res 11:1208-1211.
Conklin D, Prough R, Bhatanagar A (2006) Aldehyde metabolism in the cardiovascular system. Mol Biosyst 3:136-150.
Ebert AD, Kodo K, Liang P, Wu H, Huber BC, Riegler J, Churko J, Lee J, de Almeida P, Lan F, Diecke S, Burridge PW, Gold JD, Mochly-Rosen D, Wu JC (2014) Characterization of the molecular mechanisms underlying increased ischemic damage in the aldehyde dehydrogenase 2 genetic polymorphism using a human induced pluripotent stem cell model system. Sci Transl Med 6:255ra130.
Fan F, Sun A, Zhao H, Liu X, Zhang W, Jin X, Wang C, Ma X, Shen C, Zou Y, Hu K, Ge J (2013) MicroRNA-34a promotes cardiomyocyte apoptosis post myocardial infarction through down-regulating aldehyde dehydrogenase 2. Curr Pharm Des 19:4865-4873.
Fu SH, Zhang HF, Yang ZB, Li TB, Liu B, Lou Z, Ma QL, Luo XJ and Peng J (2014) Alda-1 reduces cerebral ischemia/reperfusion injury in rat through clearance of reactive aldehydes. Naunyn Schmiedebergs Arch Pharmacol 387:87-94.
Gao Y, Xu Y, Hua S, Zhou S and Wang K (2015) ALDH2 attenuates Dox-induced cardiotoxicity by inhibiting cardiac apoptosis and oxidative stress. Int J Clin Exp Med 8:6794-6803.
Ge W and Ren J (2012) mTOR-STAT3-notch signalling contributes to ALDH2-induced protection against cardiac contractile dysfunction and autophagy under alcoholism. J Cell Mol Med 16:616-626.
Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I (2005) G-CSF prevents cardiac remodeling aer myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med 11:305-311.
He L, Liu B, Dai Z, Zhang HF, Zhang YS, Luo XJ, Ma QL, Peng J (2012) Alpha lipoic acid protects heart against myocardial ischemia-reperfusion injury through a mechanism involving aldehyde dehydrogenase 2 activation. Eur J Pharmacol 678:32-38.
Ji W, Wei S, Hao P, Xing J, Yuan Q, Wang J, Xu F, Chen Y (2016) Aldehyde dehydrogenase 2 has cardioprotective e ff ects on myocardial ischaemia/reperfusion injury via suppressing mitophagy. Front Pharmacol 7:101.
Lee P, Sata M, Lefer DJ, Factor SM, Walsh K, Kitsis RN (2003) Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol 284:H456-463.
Li SY, Gomelsky M, Duan JH, Zhang ZJ, Gomelsky L, Zhang XC, Epstein PN, Ren J (2004) Overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells: role of ERK and p38 mitogen-activated protein kinase. J Biol Chem 279:11244-11252.
Li XG, Lin XJ, Du JH, Xu SZ, Lou XF, Chen Z (2016) Combination of methylprednisolone and rosiglitazone promotes recovery of neurological function aer spinal cord injury. Neural Regen Res 11:1678-1684.
Ma H, Guo R, Yu L, Zhang Y, Ren J (2011) Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J 32:1025-1038.
Marchitti SA, Deitrich RA, Vasiliou V (2007) Neurotoxicity and metabolism of the catecholam-inederived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol Rev 59:125-150.
Niki E, Yoshida Y, Saito Y, Noguchi N (2005) Lipid peroxidation: mechanisms, inhibition, and biological e ff ects. Biochem Biophys Res Commun 338:668-676.
Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS (2001) Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol 281: F948-957.
Pang JJ, Barton LA, Chen YG, Ren J (2015) Mitochondrial aldehyde dehydrogenase in myocardial ischemia-reperfusion injury: from bench to bedside. Sheng Li Xue Bao 67:535-544.
Renner A, Sagstetter MR, Harms H, Lange V, Götz ME, Elert O (2005) Formation of 4-hydroxy-2-nonenal protein adducts in the ischemic rat heart aer transplantation. J Heart Lung Transplant 24:430-736.
Sun A, Cheng Y, Zhang Y, Zhang Q, Wang S, Tian S, Zou Y, Hu K, Ren J, Ge J (2014) Aldehyde dehydrogenase 2 ameliorates doxorubicin-induced myocardial dysfunction through detoxi fi cation of 4-HNE and suppression of autophagy. J Mol Cell Cardiol 71:92-104.
Sun A, Zou Y, Wang P, Xu D, Gong H, Wang S, Qin Y, Zhang P, Chen Y, Harada M, Isse T, Kawamoto T, Fan H, Yang P, Akazawa H, Nagai T, Takano H, Ping P, Komuro I, Ge J (2014) Mitochondrial aldehyde dehydrogenase 2 plays protective roles in heart failure aer myocardial infarction via suppression of the cytosolic JNK/p53 pathway in mice. J Am Heart Assoc 3:e000779.
Taher F, Lebl DR, Cammisa FP, Pinter DW, Sun DY, Girardi FP (2013) Transient neurological de fi cit following midthoracic decompression for severe stenosis: a series of three cases. Eur Spine J 22: 2057-2061.
Vasiliou V, Nebert DW (2005) Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genomics 2:138-143.
Walker LM, York JL, Imam SZ, Ali SF, Muldrew KL, Mayeux PR (2001) Oxidative stress and reactive nitrogen species generation during renal ischemia. Toxicol Sci 63:143-148.
Yang T, Wu L, Wang H, Fang J, Yao N, Xu Y (2015) In fl ammation level aer decompression surgery for a rat model of chronic severe spinal cord compression and e ff ects on ischemia-reperfusion injury. Neurol Med Chir (Tokyo) 55: 578-586.
Yuan Q, Hong S, Han S, Zeng L, Liu F, Ding G, Kang Y, Mao J, Cai M, Zhu Y, Wang QX (2011) Preconditioning with physiological levels of ethanol protect kidney against ischemia/reperfusion injury by modulating oxidative stress. PLoS One 6: e25811.
Zhang P, Xu D, Wang S, Fu H, Wang K, Zou Y, Sun A, Ge J (2011) Inhibition of aldehyde dehydrogenase 2 activity enhances antimycin-induced rat cardiomyocytes apoptosis through activation of MAPK signaling pathway. Biomed Pharmacother 65:590-593.
Zhong Z, Hu Q, Fu Z, Wang R, Xiong Y, Zhang Y, Liu Z, Wang Y, Ye Q (2016) Increased expression of aldehyde dehydrogenase 2 reduces renal cell apoptosis during ischemia/reperfusion injury aer hypothermic machine perfusion. Artif Organs 40:596-603.
Zhong Z, Ye S, Xiong Y, Wu L, Zhang M, Fan X, Li L, Fu Z, Wang H, Chen M, Yan X, Huang W, Ko DS, Wang Y, Ye Q (2016) Decreased expression of mitochondrial aldehyde dehydrogenase-2 induces liver injury via activation of the mitogen-activated protein kinase pathway. Transpl Int 29:98-107.
Zhu P, Li JX, Fujino M, Zhuang J, Li XK (2013) Development and treatments of in fl ammatory cells and cytokines in spinal cord ischemia-reperfusion injury. Mediators In fl amm Article 2013:701970.
Zivin JA, DeGirolami U (1980) Spinal cord infarction: a highly reproducible stroke model. Stroke 11:200-202.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
10.4103/1673-5374.211198
Accepted: 2017-04-22
*Correspondence to: Kang-ping Shen, M.D. or Wen-jie Jin, M.D., shkp2016@163.com or surgeonjin@126.com.
- 中国神经再生研究(英文版)的其它文章
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Recovery of sympathetic nerve function after lumbar sympathectomy is slower in the hind limbs than in the torso
- Short-term observations of the regenerative potential of injured proximal sensory nerves crossed with distal motor nerves
- Long-term acupuncture treatment has a multitargeting regulation on multiple brain regions in rats with Alzheimer’s disease: a positron emission tomography study
- E ff ect of glycosides of Cistanche on the expression of mitochondrial precursor protein and keratin type II cytoskeletal 6A in a rat model of vascular dementia
- How does conserved dopamine neurotrophic factor protect against and rescue neurodegeneration of PC12 cells?

