Long-term acupuncture treatment has a multitargeting regulation on multiple brain regions in rats with Alzheimer’s disease: a positron emission tomography study
Yang-jia Lu, Xiao-wen Cai, Gui-feng Zhang, Yong Huang, Chun-zhi Tang, Bao-ci Shan, Shao-yang Cui, Jun-qi Chen, Shan-shan Qu Zheng Zhong Xin-sheng Lai Genevieve Zara Steiner
1 School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, Guangdong Province, China
2 Traditional Chinese Medicine of the Second Clinical School, Guangdong Medical College, Dongguan, Guangdong Province, China
3 Zhaoqing Medical College, Zhaoqing, Guangdong Province, China
4 Clinical Medical College of Acupuncture, Moxibustion and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong Province, China
5 Key Laboratory of Nuclear Analytical Techniques, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing, China
6 Shenzhen Hospital of Guangzhou University of Chinese Medicine, Shenzhen, Guangdong Province, China
7 Huarui Hospital of Southern Medical University, Guangzhou, Guangdong Province, China
8 National Institute of Complementary Medicine, Western Sydney University, Penrith NSW, Australia
Long-term acupuncture treatment has a multitargeting regulation on multiple brain regions in rats with Alzheimer’s disease: a positron emission tomography study
Yang-jia Lu1,2,#, Xiao-wen Cai1,#, Gui-feng Zhang3,#, Yong Huang1,*, Chun-zhi Tang4,*, Bao-ci Shan5, Shao-yang Cui4,6, Jun-qi Chen7, Shan-shan Qu1, Zheng Zhong1, Xin-sheng Lai4, Genevieve Zara Steiner8
1 School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, Guangdong Province, China
2 Traditional Chinese Medicine of the Second Clinical School, Guangdong Medical College, Dongguan, Guangdong Province, China
3 Zhaoqing Medical College, Zhaoqing, Guangdong Province, China
4 Clinical Medical College of Acupuncture, Moxibustion and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong Province, China
5 Key Laboratory of Nuclear Analytical Techniques, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing, China
6 Shenzhen Hospital of Guangzhou University of Chinese Medicine, Shenzhen, Guangdong Province, China
7 Huarui Hospital of Southern Medical University, Guangzhou, Guangdong Province, China
8 National Institute of Complementary Medicine, Western Sydney University, Penrith NSW, Australia
How to cite this article:Lu YJ, Cai XW, Zhang GF, Huang Y, Tang CZ, Shan BC, Cui SY, Chen JQ, Qu SS, Zhong Z, Lai XS, Steiner GZ (2017) Long-term acupuncture treatment has a multi-targeting regulation on multiple brain regions in rats with Alzheimer’s disease: a positron emission tomography study. Neural Regen Res 12(7):1159-1165.
Graphical Abstract
#
orcid: 0000-0003-2652-8586 (Yong Huang) 0000-0003-3127-578X (Chun-zhi Tang)
nerve regeneration; neurodegeneration; acupuncture; Zusanli (ST36); Alzheimer’s disease; long-term treatment; positron emission tomography; rat; mechanism; targeting e ff ect; compensation; multi-target regulation; neural regeneration
Introduction
Alzheimer’s disease (AD) is characterized by progressive memory loss and cognitive dysfunction. Recently, acupuncture is playing an increasingly greater complementary role in treating AD. Researchers have shown that acupuncture is safe and effective and that it can improve cognition and overall functioning, thereby improving the quality of life of AD patients (Simoncini et al., 2015; Zhou et al., 2015; Zhang et al., 2016). However, the mechanisms underlying the therapeutic e ff ects of acupuncture in AD remain unclear.
A striking feature of AD is glucose hypometabolism in brain regions related to cognition. Reductions in glucose metabolism are found in the hippocampus, bilateral precuneus and the posterior cingulate, and are correlated with symptom severity and functional decline in patients (Dukart et al., 2013; Roy et al., 2014).ese changes are also seen in cognitively normal patients with AD (Lai et al., 2015; Tahmasian et al., 2015). Furthermore, in animal models,18F-fluorodeoxyglucose (FDG) uptake is lower in several brain regions, such as the olfactory bulb, hippocampus and cerebral cortex, observed by our research group (Lu et al., 2015).ese fi ndings are similar to those of Xiao et al. (2015).erefore, in the present study, we examined cerebral glucose metabolism in the brain using18F-FDG positron emission tomography (PET) in a rat model of AD.
Acupuncture has been demonstrated to have a bene fi cial e ff ect on cognitive impairment (Li et al., 2016). Our previous studies in the rat showed that acupuncture increases blood perfusion and glucose metabolism in various brain regions, including the pyriform cortex of the left and right limbic systems, the olfactory cortex of the left and right temporal lobes, the right amygdaloid body, and the right hippocampus (Lu et al., 2014; Lai et al., 2015).
Our present study has a number of unique features. First, only one acupoint,Zusanli(ST36), was chosen for treatment. According to traditional Chinese medicine theory, a de fi ciency of spleen and stomach is the fundamental cause of AD. ST36 is an acupoint of the Stomach Meridian of Foot-Yangming, and plays an essential role in regulating the function of the spleen and stomach. In our previous report, we performed a preliminary study of acupuncture at ST36 and a non-acupoint (Lu et al., 2014). In the present study, we combined acupuncture at ST36 with PET for a clearer observation of acupuncture’s mechanism of action.
AD requires a long-term treatment because of the progressive neurodegenerative changes. Another study focused onShenmen(HT7) and showed that acupuncture at HT7 e ff ects cerebral glucose metabolism after a 30-day treatment (Lai et al., 2015). Both ST36 and HT7 are commonly used acupoints for the treatment of AD. Hence, in the current study, a 30-day treatment regimen was used with18F-FDG PET to further explore the mechanism of long-term acupuncture treatment at ST36 in a rat model of AD. Di ff erent from the previous studies which mostly focused on only one mechanism, the study hypothesized that acupuncture treated AD from several aspects.
Materials and Methods
Experimental animals
Seventy-five 2-month-old Sprague-Dawley rats (38 males and 37 females) weighing 200–250 g were provided by the Animal Center of China Academy of Chinese Medical Sciences (Beijing, China; license No. SCXK (Jing) 2011-0007). The study protocol was approved by the Ethics Committee of Guangzhou University of Chinese Medicine (No. SPF20110032). The experimental procedure followed the the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986), and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association for Veterinary Editors (IAVE).
AD model establishment
Several studies have shown the feasibility of intraperitoneal injection of D-galactose (Shanghai No. 2 Reagent Company, Shanghai, China) combined with injection of ibotenic acid (Sigma-Aldrich, St. Louis, MO, USA) into the basal nuclei to generate a rat model of AD (Budni et al., 2016; Karthick et al., 2016). D-galactose accelerates the natural aging process, which contributes to the pathogenesis of AD. Ibotenic acid impairs the long-term memory of rats.
ST36 and sham point locations
ST36 is located 5 mm directly below the capitulum fi bulae, according toAcupoint Location for Experimental Animals(Hu, 2003). Based on a previous study (Lin et al., 2013), the selected sham point is 1.5 mm distal to the ST36 point. In this experiment, we used points on the right side of the rats.
Acupuncture treatment
Y-maze test
Learning and memory abilities were tested using the Y-maze test system (Zhenhua Teaching Instrument Factory, Yuanyang, China). A correct response was noted only if the rat directly moved into the secure arm of the Y-maze when it received a foot shock (0.6 mA).e rats were trained until they had reached the learning criterion; namely, having 9 correct responses within 10 consecutive training trials.e Y-maze test results were calculated as the time required by the rats to reach the learning criterion.e shorter the time required for training, the greater the capacity for learning. The tests were carried out by professionals in a quiet and dim room, with a fi xed training time.
18F-FDG-PET imaging
On day 31, all of the rats were sent to the PET-CT Center of the Experimental Animals Center of the No. 301 Chinese People’s Liberation Army General Hospital aer 24 hours of fasting.e rats underwent the following procedure: (1)e blood sugar level was determined; (2)e rats were allowed to rest for 20 minutes in a dark room; (3) A tracer (18F-FDG, synthesized with Mini Tracer accelerator, 0.11 mci/kg dosage, PET-CT Center of the Experimental Animals Center of the No. 301 Chinese PLA General Hospital, Beijing, China) was injectedviathe tail vein; (4)e rats were allowed to rest for 40 minutes; (5)e rats were subjected to PET scans (Siemens, Germany). PET scans were performed on a Biograph Duo BGO scanner (Siemens).e images encompassed the whole brain and neck.e matrix size of the PET images was 128 × 128 × 63, and the format was ANALYZE 7.5.
PET image post-processing was performed using the pixel statistics analysis soware SPM2, based on the Matlab platform (the Institute of High Energy Physics of the Chinese Academy of Sciences, Beijing, China). Pre-processing and data analysis were performed using our self-developed toolbox for voxel-wise analysis of rat brain images based on SPM8 (Welcome Department of Cognitive Neurology, London, UK), which comprised a FDG-PET rat brain template and atlas in Paxinos & Watson space (Nie et al., 2013). The images of all rats in the model, sham-point, ST36 and normal groups were pre-processed using the following main steps: (1) segmentation; (2) normalization; (3) smoothing.
Statistical analysis and image processing
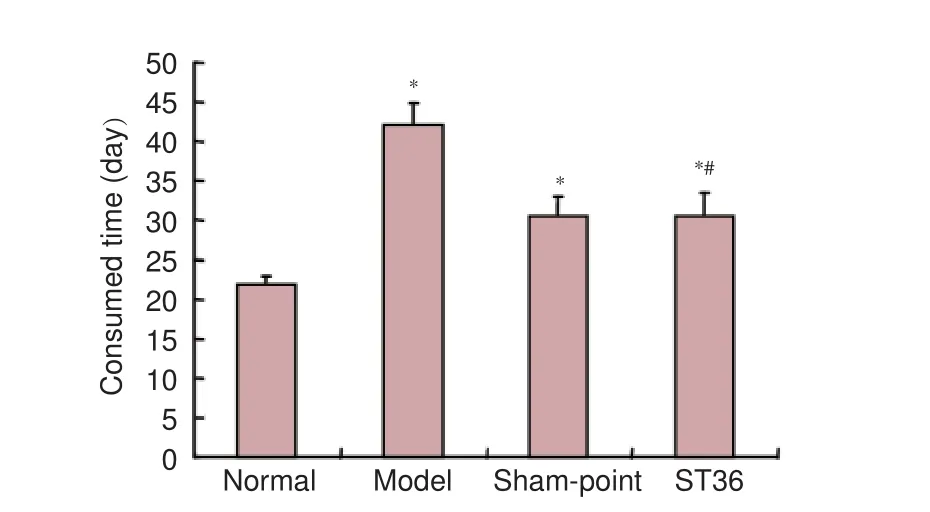
Figure 1 Learning and memory skills assessed by Y-maze test.
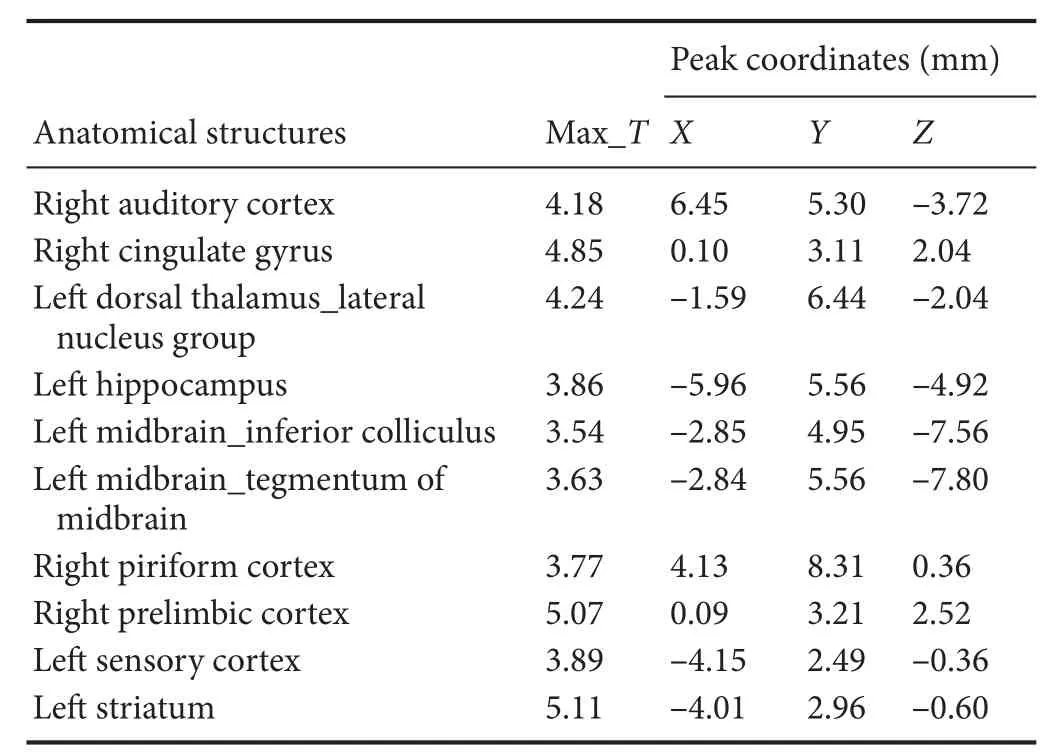
Table 1 Increased glucose metabolism in di ff erent brain regions in the normal group compared with the model group
Y-maze data were analyzed by one-way analysis of variance and the least signi fi cant di ff erence test, and were expressed as the mean ± SD and processed with the Statistical Package for the Social Sciences version 13.0 soware (SPSS, Chicago, IL, USA). A value ofP< 0.05 was considered statistically signi fi cant.
Results
E ff ects of long-term acupuncture at ST36 on learning and memory abilities in AD rats
After acupuncture, the ST36 and sham-point groups displayed a significant improvement in learning and memory functions, evidenced by the reduction in the length of time required for training to achieve the learning criterion in the Y-maze test (Figure 1).
E ff ects of long-term acupuncture at ST36 on multiregional brain activation in AD rats
Compared with the model group, the normal group exhibited a higher level of glycol metabolism in the right auditory cortex, right cingulate gyrus, lelateral nucleus group of the dorsal thalamus, left hippocampus, left inferior colliculus and tegmentum of the midbrain, right piriform cortex, right prelimbic cortex, left sensory cortex, and the left striatum (Table 1 and Figure 2). Compared with the model group, the ST36 group exhibited a higher level of glycol metabolism in the lehippocampus, leinfralimbic cortex, lemedullaoblongata, leinferior colliculus of the midbrain, leolfactory cortex, leorbital cortex, letegmentum of the pons, and the leprelimbic cortex (Table 2 and Figure 3). Compared with the sham-point group, the ST36 group showed a higher level of glycol metabolism in the leanterior lobe and posterior lobe of the cerebellum, lebasilar part of the pons, and the letegmentum of the pons (Table 3 and Figure 4). Compared with the model group, the sham-point group showed a higher level of glycol metabolism in the right auditory cortex, leinfralimbic cortex, leorbital cortex, leprelimbic cortex and right sensory cortex (Table 4 and Figure 5). Compared with the normal group, the ST36 group showed a higher level of glycol metabolism in the leamygdaloid body, right anterior lobe, lecerebellar nucleus and leposterior lobe of the cerebellum, the lepiriform cortex, and the letegmentum of the pons (Table 5 and Figure 6). Compared with the normal group, the sham-point group displayed a higher level of glycol metabolism in the right anterior lobe and right posterior lobe of the cerebellum, lehippocampus, right infralimbic cortex, lesuperior colliculus of the midbrain, right orbital cortex, and the right visualcortex (Table 6 and Figure 7).
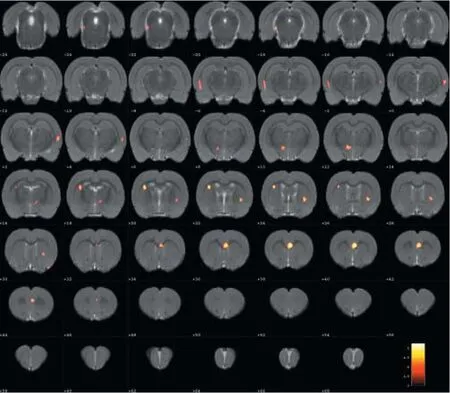
Figure 2 Changes in glucose metabolism in di ff erent brain regions in the normal group compared with the model group (coronal view).
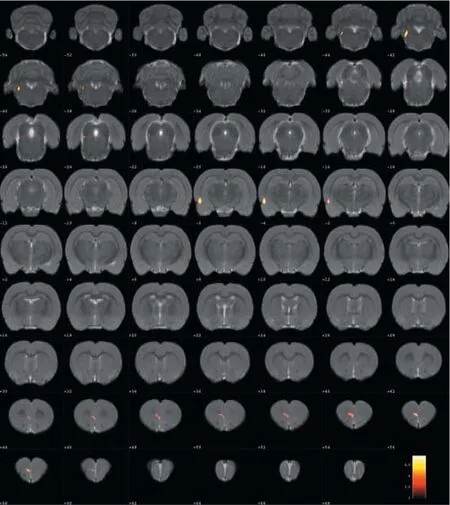
Figure 3 Changes in glucose metabolism in di ff erent brain regions in theZusanli (ST36) group compared with the model group (coronal view).

Figure 4 Changes in glucose metabolism in di ff erent brain regions in theZusanli(ST36) group compared with the sham-point group (coronal view).
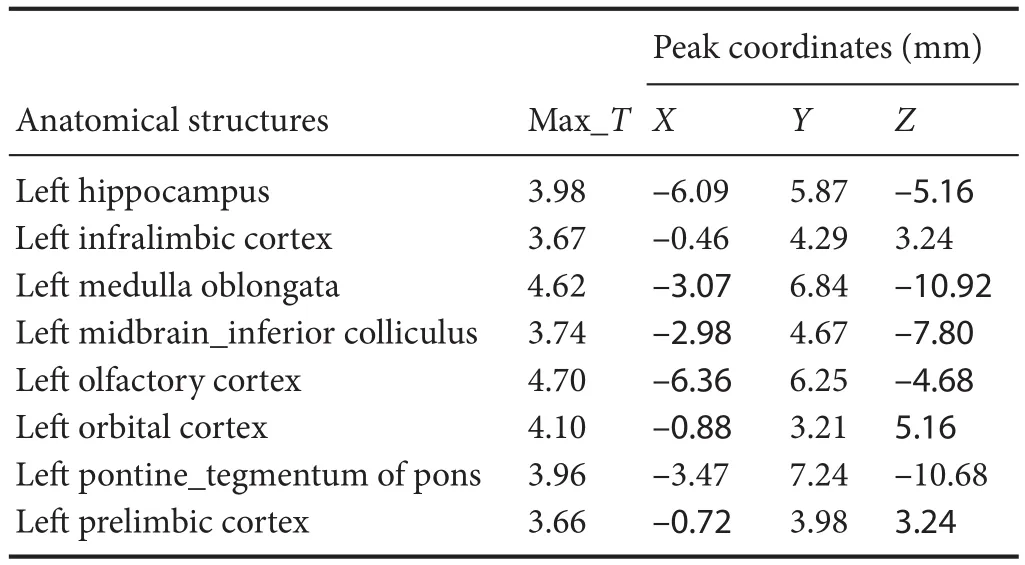
Table 2 Increased glucose metabolism in di ff erent brain regions in theZusanli(ST36) group compared with the model group
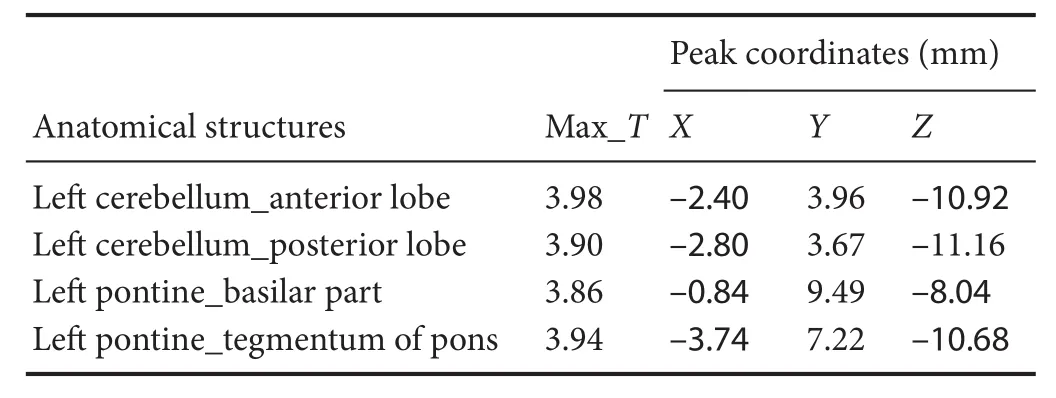
Table 3 Increased glucose metabolism in di ff erent brain regions in theZusanli(ST36) group compared with the sham-point group
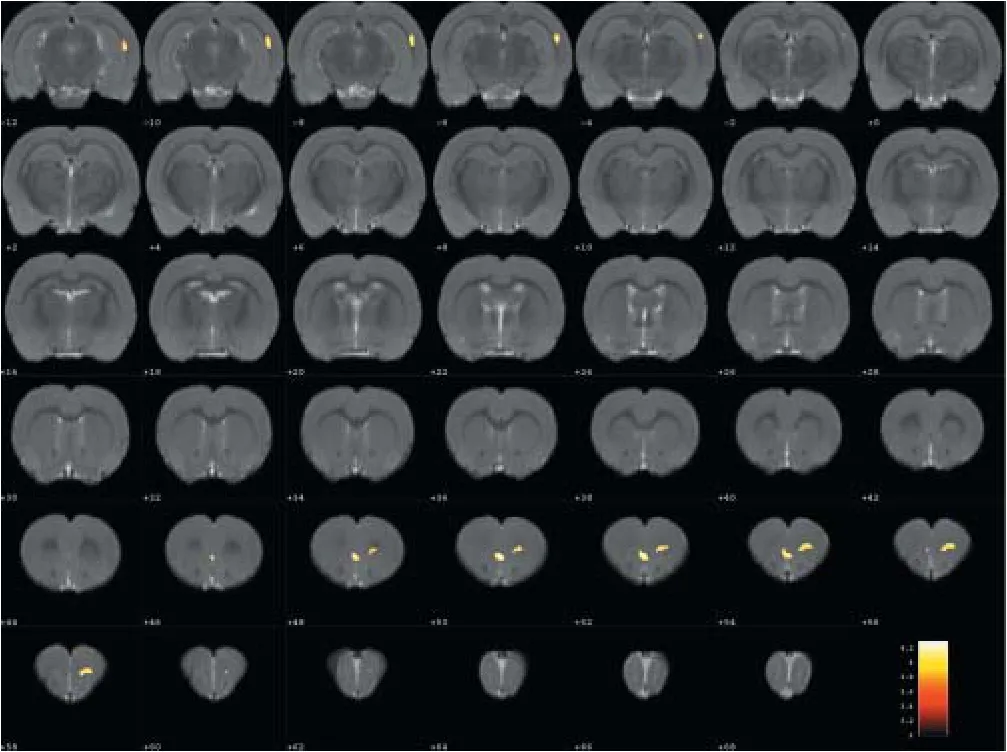
Figure 5 Changes in glucose metabolism in di ff erent brain regions in the sham-point group compared with the model group (coronal view).

Figure 6 Changes in glucose metabolism in di ff erent brain regions in theZusanli(ST36) group compared with the normal group (coronal view).
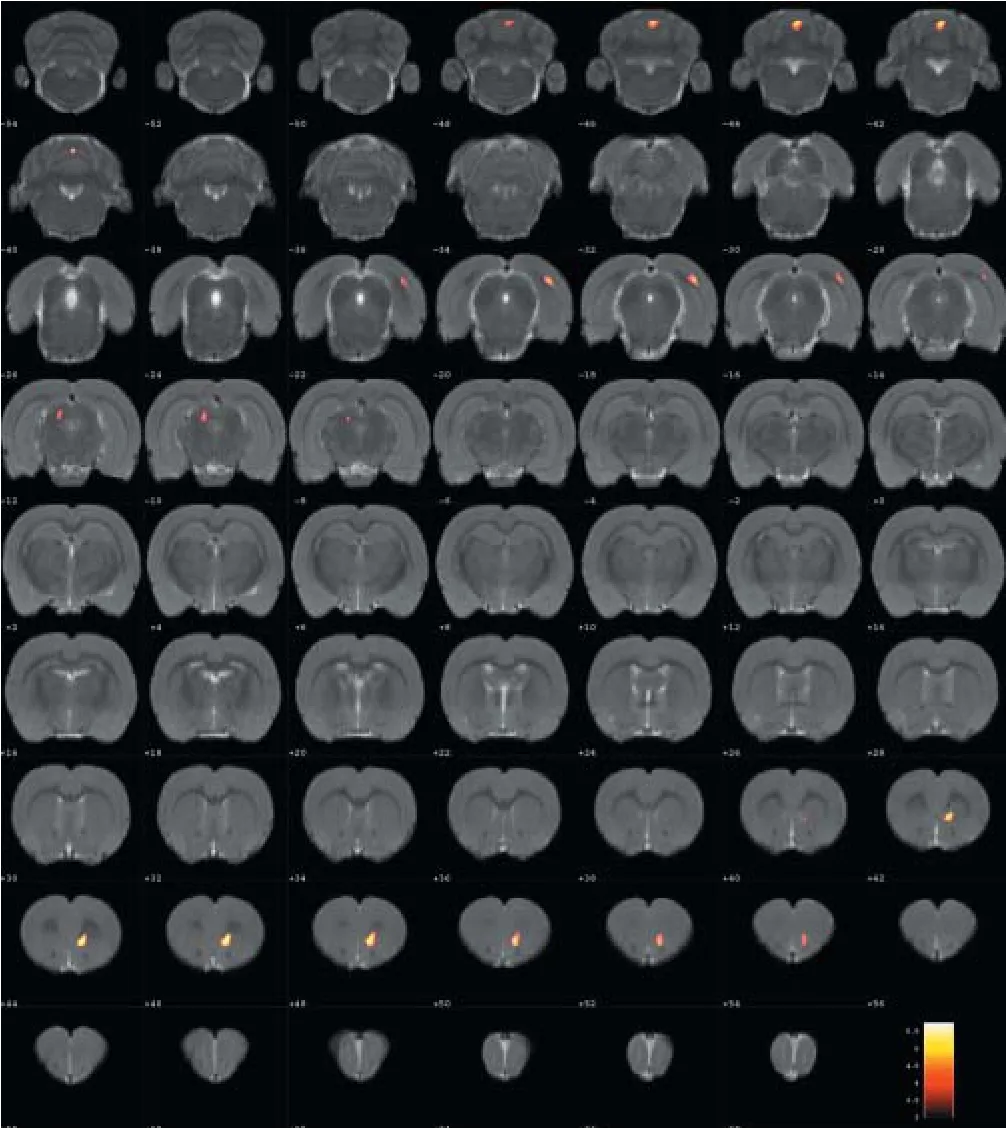
Figure 7 Changes of in glucose metabolism in di ff erent brain regions in the sham-point group compared with the normal group (coronal view).
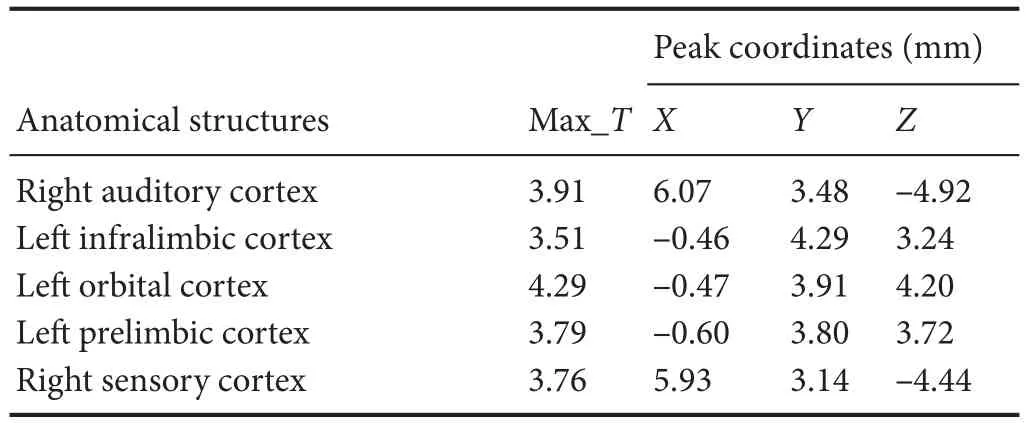
Table 4 Increased glucose metabolism in di ff erent brain regions in the sham-point group compared with the model group
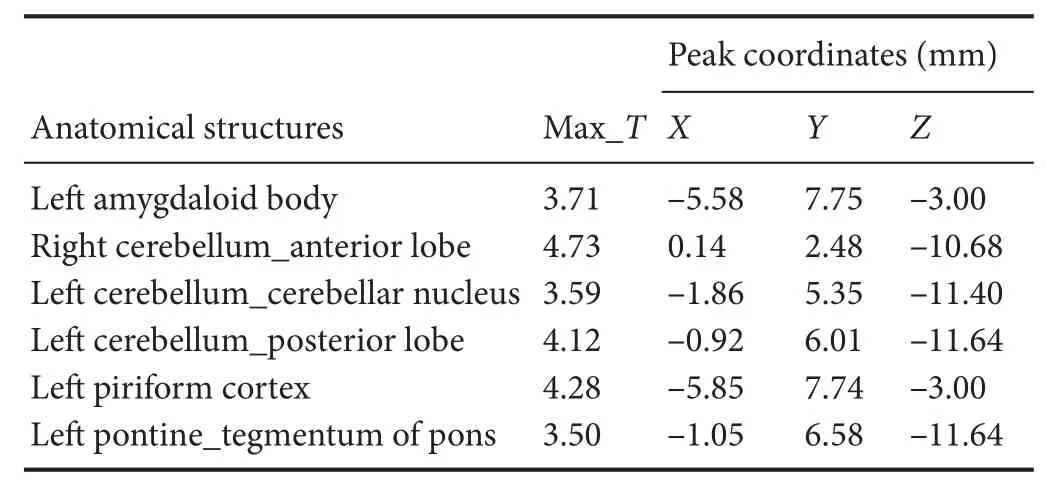
Table 5 Increased glucose metabolism in di ff erent brain regions in theZusanli(ST36) group compared with the normal group
Discussion
Our results demonstrate that long-term acupuncture treatment has therapeutic e ffi cacy for AD. Rats in the ST36 group fi nished the Y-maze test in a shorter time compared with the sham-point and model groups, suggesting an improvement of symptoms.18F-FDG PET was applied in the study to provide insight into the underlying mechanisms. Memory disorder, one of the prominent features of AD, is re fl ected pathologically as a reduction in glucose metabolism in several brain regions of AD rats compared with the normal group.ese regions included the hippocampus, the tegmentum of the midbrain, the prelimbic cortex, the auditory cortex, the striatum and the cingulate gyrus. ST36 is a key acupoint in acupuncture treatment for many brain diseases, especially AD. Aer needling at ST36, glucose metabolism was increased in brain regions in the lehippocampus and the leprelimbic cortex.ese changes are evidence of a targeted e ff ect of acupuncture on multiple brain areas. Needling at ST36 also stimulated other brain regions, including the left infralimbic cortex, the left medulla oblongata, the left olfactory cortex, the left orbital cortex and the letegmentum of the pons. We speculate that these e ff ects of acupuncture enhance brain function and ameliorate cognitive impairment, in line with the study by Wang et al. (2012).
In addition to cognitive impairment, a number of AD patients also su ff er from behavioral and psychiatric symptoms, among which agitation and depression are the most common (Tsai et al., 2013). In our experiment, the orbital cortex, the medulla oblongata and the tegmentum of the pons were activated aer needling at ST36. As a part of the prefrontal cortex, the orbital cortex is considered of vital importance in the control of emotions. When damaged, this control, especially the inhibition of impulsivity, can be perturbed (Kuo et al., 2015). AD patients can also exhibit disturbances in eating and circadian rhythms (Kai et al., 2015; Musiek et al., 2015), which are related to the medulla oblongata and the tegmentum of the pons. As AD progresses, some patients may experience motor system dysfunction, which is also associated with pontine activity (Lam et al., 2013; Takakusaki et al., 2016). In the present study, the letegmentum of the pons was activated after needling at ST36 in both the normal and model groups, with a larger increase in the latter.is suggests that acupuncture at ST36 may be e ff ective for treating AD. Although studies on the e ff ects of acupuncture on non-cognitive symptoms are lacking, acupuncture might produce an overall improvement in the disease.
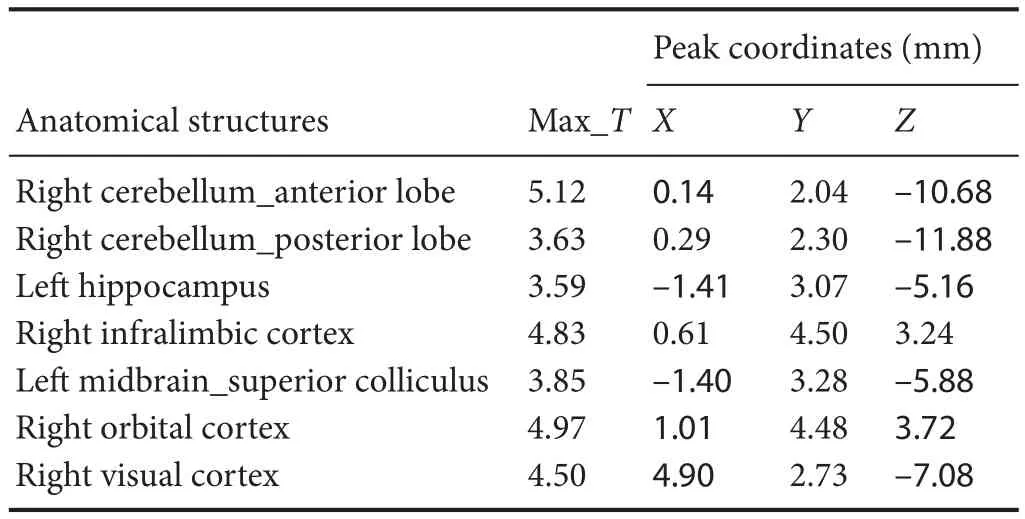
Table 6 Increased glucose metabolism in di ff erent brain regions in the sham-point group compared with the normal group
The sham-point group shared some similarities with the ST36 group, compared with the model group, as both groups exhibited an upregulation of glucose metabolism of the left infralimbic cortex, left orbital cortex and left prelimbic cortex.e Y-maze test results were also similar among the sham-point and ST36 groups. One possible reason for these similarities is that ST36 and the sham-point are part of the same nerve segment (Ren et al., 2014). When needling at ST36 or the sham point, the brain regions connected with these nerve segments were activated. Furthermore, the locations of ST36 and the sham point are close, particularly in the rat.e brain regions activated in both the ST36 and sham-point groups included the lecerebellum and the lepons. Moreover, the sham-point stimulation activated the right auditory cortex and the right sensory cortex. Damage to the auditory cortex can be reflected in verbal episodic memory impairment in AD (Dhanjal et al., 2013). Therefore, an increase in metabolism could account for the targeted therapeutic e ff ect of acupuncture. In addition, it is reported that nearly half of AD patients have hallucinations (Murray et al., 2014).is might be related to dysfunction of the sensory cortex. PET observation has not been previously reported for needling at the sham point for treating AD, and therefore requires further study.
Acknowledgments:We wish to acknowledge the co-operation of the Experimental Animals Center of China Academy of Chinese Medical Sciences. Meanwhile, we are very grateful to the help of Bin-bin Nie whoassisted the data analysis and all those who generously contributed their time and e ff orts on this experiment and the manuscript.
Author contributions:YH, CZT and XSL conceived and coordinated the study. YH and YJL participated in the design of the study. YJL, SYC and JQC performed the study. BCS performed the data analysis with SPM2. SSQ, ZZ, XWC, GZS and GFZ helped to record the data. XWC wrote the paper. All authors read and approved the fi nal version of the paper.
Con fl icts of interest:None declared.
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Budni J, Pacheco R, Da Silva S, Garcez ML, Mina F, Bellettini-Santos T, de Medeiros J, Voss BC, Steckert AV, Valvassori Sda S, Quevedo J (2016) Oral administration of d-galactose induces cognitive impairments and oxidative damage in rats. Behav Brain Res 302:35-43.
Dhanjal NS, Warren JE, Patel MC, Wise RJ (2013) Auditory cortical function during verbal episodic memory encoding in Alzheimer’s disease. Ann Neurol 73:294-302.
Dukart J, Mueller K, Villringer A, Kherif F, Draganski B, Frackowiak R, Schroeter ML (2013) Relationship between imaging biomarkers, age, progression and symptom severity in Alzheimer’s disease. Neuroimage Clin 3:84-94.
Hu Y (2003) Acupoint location for experimental animals. Beijing: China Agriculture Press.
Kai K, Hashimoto M, Amano K, Tanaka H, Fukuhara R, Ikeda M (2015) Relationship between eating disturbance and dementia severity in patients with Alzheimer’s disease. PLoS One doi:10.1371/journal. pone.0133666.
Karthick C, Periyasamy S, Jayachandran KS, Anusuyadevi M (2016) Intrahippocampal administration of ibotenic acid induced cholinergic dysfunction via NR2A/NR2B expression: implications of resveratrol against alzheimer disease pathophysiology. Front Mol Neurosci 9:28.
Kuo MF and Nitsche MA (2015) Exploring prefrontal cortex functions in healthy humans by transcranial electrical stimulation. Neurosci Bull 31:198-206.
Lai X, Ren J, Lu Y, Cui S, Chen J, Huang Y, Tang C, Shan B, Nie B (2015) E ff ects of acupuncture at HT7 on glucose metabolism in a rat model of Alzheimer’s disease: an18F-FDG-PET study. Acupunct Med doi:10.1136/acupmed-2015-010865.
Lam B, Masellis M, Freedman M, Stuss DT, Black SE (2013) Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimers Reser 5:1.
Li W, Kong LH, Wang H, Shen F, Wang YW, Zhou H, Sun GJ (2016) High-frequency electroacupuncture evidently reinforces hippocampal synaptic transmission in Alzheimer’s disease rats. Neural Regen Res 11:801-806.
Lin Y, Ji F, Huang GR, Li P (2013) Our considerations about non-acupoint selection for experimental studies in rats. Zhen Ci Yan Jiu 38:334-338.
Lu Y, Huang Y, Tang C, Shan B, Cui S, Yang J, Chen J, Lin R, Xiao H, Qu S, Lai X (2014) Brain areas involved in the acupuncture treatment of AD model rats: a PET study. BMC Complement Altern Med 14:178.
Lu Y, Ren J, Cui S, Chen J, Huang Y, Tang C, Shan B, Nie B, Lai X (2015) Cerebral glucose metabolism assessment in rat models of alzheimer’s disease: an 18F-FDG-PET study. Am J Alzheimers Dis Other Demen 31:333-340.
Murray PS, Kumar S, Demichele-Sweet MA, Sweet RA (2014) Psychosis in Alzheimer’s disease. Biol Psychiatry 75:542-552.
Musiek ES, Xiong DD and Holtzman DM (2015) Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med doi:10.1038/emm.2014.121.
Nie B, Chen K, Zhao S, Liu J, Gu X, Yao Q, Hui J, Zhang Z, Teng G, Zhao C, Shan B (2013) A rat brain MRI template with digital stereotaxic atlas of fine anatomical delineations in paxinos space and its automated application in voxel-wise analysis. Hum Brain Mapp 34:1306-1318.
Ren BB, Yu Z, Wang YL, Xu B (2014) Regulatory e ff ect of electroacupuncture on heart and stomach of rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 34:1212-1215.
Roy K, Pepin LC, Philiossaint M, Lorius N, Becker JA., Locascio JJ, Rentz DM, Sperling RA, Johnson KA, Marshall GA (2014) Regional fl uorodeoxyglucose metabolism and instrumental activities of daily living across the Alzheimer’s disease spectrum. J Alzheimers Dis 42:291-300.
Simoncini M, Gatti A, Quirico PE, Balla S, Capellero B, Obialero R, D’Agostino S, Sandri N, Pernigotti LM (2015) Acupressure in insomnia and other sleep disorders in elderly institutionalized patients su ff ering from Alzheimer’s disease. Aging Clin Exp Res 27:37-42.
Tahmasian M, Pasquini L, Scherr M, Meng C, Forster S, Mulej Bratec S, Shi K, Yakushev I, Schwaiger M, Grimmer T, Diehl-Schmid J, Riedl V, Sorg C, Drzezga A (2015)e lower hippocampus global connectivity, the higher its local metabolism in Alzheimer disease. Neurology 84:1956-1963.
TakakusakiK, Chiba R, Nozu T, Okumura T (2016) Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J Neural Transm (Vienna) 123:695-729.
Tsai CF, Hung CW, Lirng JF, Wang SJ, Fuh JL (2013) Differences in brain metabolism associated with agitation and depression in Alzheimer’s disease. East Asian Arch Psychiatry 23:86-90.
Wang Z, Nie B, Li D, Zhao Z, Han Y, Song H, Xu J, Shan B, Lu J, Li K (2012) Effect of acupuncture in mild cognitive impairment and Alzheimer disease: a functional MRI study. PLoS One doi:10.1371/ journal.pone.0042730.
Xiao N, Zhang J, Zhou M, Wei Z, Wu X, Dai X, Zhu Y, Chen X (2015) Reduction of glucose metabolism in olfactory bulb is an earlier alzheimer’s disease-related biomarker in 5XFAD mice. Chin Med J (Engl) 128:2220-2227.
Zhang S, Huang XY, Liu S, Li YJ, Zhao JC (2016) E ff ects of amyloid-beta 25-35 on expression of synapse-associated proteins in PC12 neurons E ff ects of amyloid-beta 25-35 on expression of synapse-associated proteins in PC12 neurons. Zhongguo Zuzhi Gongcheng Yanjiu 20:224-229.
Zhong Z, Qu Z, Wang N, Wang J, Xie Z, Zhang F, Zhang W, Lu Z (2005a) Protective e ff ects of Panax notoginseng saponins against pathological lesion of cholinergic neuron in rat model with Alzheimer’s disease. Zhong Yao Cai 28:119-122.
Zhong Z, Qu Z, Wang N, Zhang F, Zhang W, Lu U (2005b) E ff ects of the Panax notoginseng saponins on the level of synaptophysin protein in brain in rat model with lesion of Meynert. Zhongguo Zhong Yao Za Zhi 30:913-915.
Zhou J, Peng W, Xu M, Li W, Liu Z (2015)e e ff ectiveness and safety of acupuncture for patients with Alzheimer disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 94:e933.
Copyedited by Patel B, Wysong S, Wang J, Li CH, Qiu Y, Song LP, Zhao M
10.4103/1673-5374.211197
Accepted: 2017-05-24
*Correspondence to: Yong Huang, Ph.D. or Chun-zhi Tang, Ph.D., nanfanglihuang@163.com or jordan664@163.com.
- 中国神经再生研究(英文版)的其它文章
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Recovery of sympathetic nerve function after lumbar sympathectomy is slower in the hind limbs than in the torso
- Short-term observations of the regenerative potential of injured proximal sensory nerves crossed with distal motor nerves
- Aldehyde dehydrogenase 2 overexpression inhibits neuronal apoptosis after spinal cord ischemia/ reperfusion injury
- E ff ect of glycosides of Cistanche on the expression of mitochondrial precursor protein and keratin type II cytoskeletal 6A in a rat model of vascular dementia
- How does conserved dopamine neurotrophic factor protect against and rescue neurodegeneration of PC12 cells?

