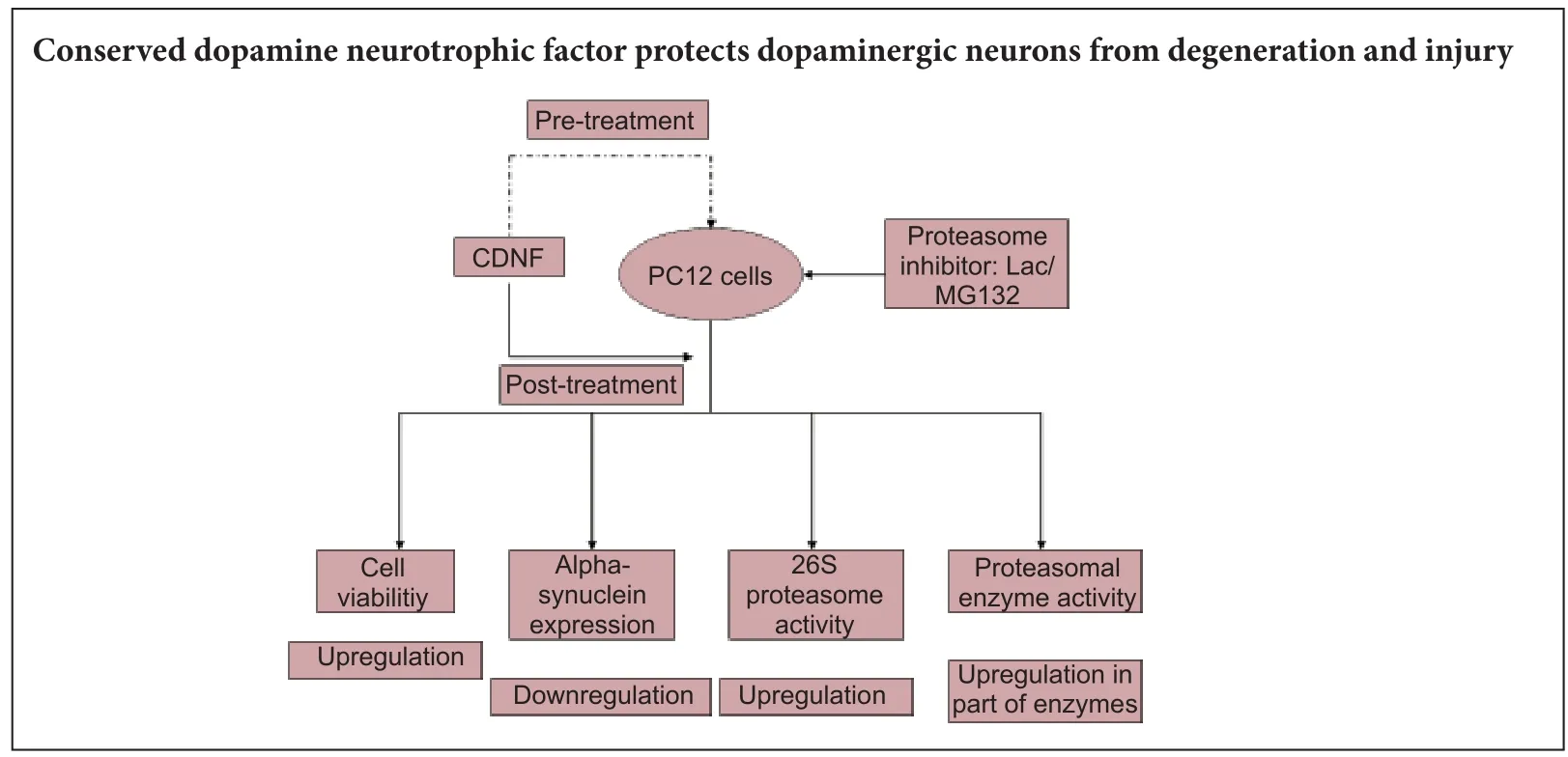
How does conserved dopamine neurotrophic factor protect against and rescue neurodegeneration of PC12 cells?
Jia-ming Mei, Chao-shi Niu,
1 Shandong University, Jinan, Shandong Province, China
2 Department of Neurosurgery, Anhui Provincial Hospital, Hefei, Anhui Province, China
How does conserved dopamine neurotrophic factor protect against and rescue neurodegeneration of PC12 cells?
Jia-ming Mei1,2, Chao-shi Niu1,2,*
1 Shandong University, Jinan, Shandong Province, China
2 Department of Neurosurgery, Anhui Provincial Hospital, Hefei, Anhui Province, China
How to cite this article:Mei JM, Niu CS (2017) How does conserved dopamine neurotrophic factor protect against and rescue neurodegeneration of PC12 cells? Neural Regen Res 12(7):1145-1151.
Graphical Abstract
orcid: 0000-0002-5429-1443 (Chao-shi Niu)
Conserved dopamine neurotrophic factor protects and rescues dopaminergic neurodegeneration induced by 6-hydroxydopaminein vivo, but its potential value in treating Parkinson’s disease remains controversial. Here, we used the proteasome inhibitors lactacystin and MG132 to induce neurodegeneration of PC12 cells. Aerwards, conserved dopamine neurotrophic factor was administrated as a therapeutic factor, both pretreatment and posttreatment. Our results showed that (1) conserved dopamine neurotrophic factor enhanced lactacystin/MG132-induced cell viability and morphology, and attenuated alpha-synuclein accumulation in di ff erentiated PC12 cells. (2) Enzyme linked immunosorbent assay showed up-regulated 26S proteasomal activity in MG132-induced PC12 cells after pre- and posttreatment with conserved dopamine neurotrophic factor. Similarly, 26S proteasome activity was upregulated in lactacystin-induced PC12 cells pretreated with conserved dopamine neurotrophic factor. (3) With regard proteolytic enzymes (speci fi cally, glutamyl peptide hydrolase, chymotrypsin, and trypsin), glutamyl peptide hydrolase activity was up-regulated in lactacystin/MG132-administered PC12 cells aer pre- and posttreatment with conserved dopamine neurotrophic factor. However, upregulation of chymotrypsin activity was only observed in MG132-administered PC12 cells pretreated with conserved dopamine neurotrophic factor.ere was no change in trypsin expression. We conclude that conserved dopamine neurotrophic factor develops its neurotrophic e ff ects by modulating proteasomal activities, and thereby protects and rescues PC12 cells against neurodegeneration.
nerve regeneration; conserved dopamine neurotrophic factor; Parkinson’s disease; proteasomal inhibitor; 26S proteasome; alphasynuclein; lactacystin; MG-132; glutamyl peptide hydrolase; chymotrypsin; trypsin; neural regeneration
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder. It is primarily characterized pathologically by progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta, with accumulation of alpha-synuclein protein inclusions in surviving neurons. Despite this recognized pathology, its inherent pathogenesis remains elusive. Oxidative stress can cause neurodegeneration of DA neurons (Anusha et al., 2017; Fatkullina et al., 2017). However, numerous studies have demonstrated that ubiquitin-proteasomal system dysfunction plays a key role in PD pathogenesis, bothin vitroandin vivo(Bentea et al., 2015; Wang et al., 2015; Chu et al., 2016).
The proteasome is a multienzyme complex that exhibits multienzymatic proteolytic activities of chymotrypsin- and trypsin-like hydrolases, and is implicated in degeneration ofabnormal proteins in eukaryotic cells. McNaught et al. reported impaired proteasomal function in the substantia nigra of PD patients in autopsy brain tissue (McNaught and Jenner, 2001; McNaught et al., 2006). Further studies found that proteasome inhibition by striatal and nigral injections orin vivosystemic administration causes abnormal behavior and neurodegeneration of substantia nigra DA neurons (McNaught et al., 2002, 2004; Bedford et al., 2008), which causes typical pathological featuresin vitro(Rideout et al., 2005; Nair et al., 2006).ese studies have provided new insight into the rat model of PD after proteasome inhibition.
Conserved dopamine neurotrophic factor (CDNF) is a member of the mammalian mesencephalic astrocyte-derived neurotrophic factor family that signi fi cantly protects and prevents neurodegeneration of DA neurons from 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced damage, and has potential as a candidate neurotrophic therapy for PD (Lindholm et al., 2007; Voutilainen et al., 2011; Airavaara et al., 2012; Bäck et al., 2013). As far as oxidative stress is concerned, proteasomal dysfunction is a factor in PD pathogenesis, and consequently, it is of interest to determine similar effects of CDNF against DA neurodegeneration induced by proteasomal inhibitors.
In the present study, we aimed to determine whether CDNF exhibits comparable neuroprotective and reversal effects in proteasomal inhibitor-treated PC12 cells, and examine the underlying mechanism involved in proteasomal expression and its multienzymatic proteolytic activities.
Materials and Methods
CDNF extraction and cell culture
Human recombinant CDNF proteins were produced and purified as previously described (Lindholm et al., 2007). PC12 cells were directly obtained from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China), and were maintained in Dulbecco’s modi fi ed Eagle’s medium (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) supplemented with 10% heat-inactivated newborn calf serum. Cells were cultured at 37°C in humidified air with 5% CO2. All experiments were performed within 24–48 hours aer cell seeding.
Cell treatment
Viability of PC12 cells by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay
After 24 hours, cell viability was measured using the MTT assay. Cells were washed once with phosphate bu ff ered saline (PBS) before adding 0.1 mL serum-free medium containing 1 mg/mL MTT (Sigma-Aldrich Corp.) to each well. After incubation for 3 hours, the supernatant was removed and obtained formazan product dissolved in 150 μL dimethyl sulfoxide (Sigma-Aldrich Corp.) per well, with stirring for 15 minutes on a microtiter plate shaker. Absorbance values were recorded at 570 nm.
Immuno fl uorescence staining for tyrosine hydroxylase (TH) and alpha-synuclein
After scheduled treatment for 24 hours, PC12 cells were permeabilized and fi xed in 4% paraformaldehyde and 0.5% Triton X-100. Slides were blocked with 1% normal donkey serum (Merck, Darmstadt, Germany) in PBS for 30 minutes at room temperature. Cells were washed three times with gentle shaking in 0.1% bovine serum albumin (Beyotime Institute of Biotechnology, Shanghai, China) in PBS, and then incubated with primary antibodies diluted in 0.1% bovine serum albumin/PBS (TH, 1:500; and alpha-synuclein, 1:200; anti mouse monoclonal antibodies; Invitrogen, Paisley, UK) at 4°C overnight. Labeled donkey anti-rabbit immunoglobulin G (IgG) (1:1,000; Invitrogen) was used as the secondary antibody, with the solution incubated in the dark for 2 hours at room temperature. Speci fi c antibody binding was detected by Alexa Fluor 488 (green label)-conjugated ExtrAvidin (Sigma-Aldrich Corp.). Fluorescence density was quanti fi ed. Fluorescence microscopy was performed using an Olympus BX51 200 M fl uorescent microscope (Olympus, Tokyo, Japan).
Measurement of 26S proteasomal activity
Hydrolase enzymatic activity assays
Cells were lysed in 10 μM digitonin in Tris bu ff er (50 mM Tris-HCl, 1 mM ethylenediaminetetraacetic acid, and 10 mM ethylene glycol tetraacetic acid). Lysate supernatants were obtained by centrifugation at 14,000 ×gfor 5 minutes, and then incubated with 50 μL fl uorogenic substrates (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) for determination of activity of glutamyl peptide hydrolase, chymotrypsin, and trypsin (Sigma-Aldrich Corp.). Colored reagents A and B were added successively at 37°C for 15 minutes. Absorbance (optical density) values were measured at 450 nm using an enzyme-linked immunosorbent assay kits of glutamyl peptide hydrolase, chymotrypsin and trypsin (Chemicon International, Temecula, CA, USA).
Statistical analysis
All data are expressed as the mean ± SEM. Statistical analysis was performed using one-way analysis of variance and Student-Newman-Keuls test.P-values of 0.05 or less were considered statistically signi fi cant.
Results
PC12 cell viability aer pre- and posttreatment of CDNF
Expression of alpha-synuclein measured by immuno fl uorescence staining
Compared with controls, alpha-synuclein immunofluorescence was observed in numerous lactacystin- and MG132-treated PC12 cells with high fl uorescence expression (Figure 2B, C). Fluorescence intensity in lactacystin- and MG132-treated PC12 cells was quantified as 2.1755 and 2.8990, respectively (Figure 2H, I). Aer pretreatment with 200 nM CDNF protein for 6 hours, the number of immunofl uorescent PC12 cells was reduced, and fl uorescence intensity faded (Figure 2D, E). Speci fi cally, fl uorescence intensity in lactacystin- and MG132-treated PC12 cells decreased to 1.3871 and 1.1710, respectively (Figure 2H). In samples posttreated with CDNF, the number of cells and fl uorescence intensity also decreased (Figure 2F, G), with fluorescence intensity in lactacystin- and MG132-treated PC12 cells decreased to 1.4899 and 1.2958, respectively (Figure 2I).
26S proteasome activity results
After exposure to lactacystin and MG132, 26S proteasome activity was significantly decreased (P< 0.05) (Figure 3A, B). Aer CDNF pretreatment, 26S proteasome activity was upregulated in the lactacystin and MG132 groups (P< 0.05), with a greater e ff ect in the MG132 group (P< 0.05) (Figure 3A). Aer CDNF posttreatment, upregulation was observed in the MG132 group (P< 0.05), but not the lactacystin group (P> 0.05) (Figure 3B).
Hydrolase enzymatic activity
Hydrolase enzymatic activity was determined, as described previously (Xie et al., 2010). After exposure to lactacystin and MG132, activities of glutamyl peptide hydrolase, chymotrypsin, and trypsin were significantly decreased (P<0.05) (Figure 4A−F). Aer CDNF pretreatment, upregulation of glutamyl peptide hydrolase and chymotrypsin was observed in the MG132 group (P< 0.05) (Figure 4A−C). Only glutamyl peptide hydrolase expression was increased in the lactacystin group (P< 0.05). No statistical di ff erences in activity were related to trypsin (P> 0.05). Finally, in the group pretreated with lactacystin and MG132, only glutamyl peptide hydrolase was reversibly up-regulated compared with the other enzymes (Figure 4D–F).
Discussion
Here, we show for the fi rst time that CDNF exhibits a neuroprotective and reversible effect on proteasomal inhibitor-treated (lactacystin and MG132) PC12 cellsviaupregulation of cell viability, reduction of alpha-synuclein protein, and upregulation of the 26S proteasome and its corresponding proteolytic activity.
Although PD pathogenesis encompasses multiple factors, the ubiquitin-proteasomal system is one of the major intracellular proteolysis systems, responsible for degradation of damaged or misfolded proteins including those involved in various cellular processes such as neurodegeneration. Emerging evidence has shown that failure of the ubiquitin-proteasomal system to degrade aberrant proteins plays a key role in PD pathogenesis (Jnobaptiste et al., 2002; Mc-Naught et al., 2010; Xie et al., 2010). In our previous study, we found that accumulation of misfolded and aggregated alpha-synuclein aer ubiquitin-proteasomal system impairment led to degeneration of DA neurons in the substantia nigra pars compacta of rats (Niu et al., 2009). Once proteasomal dysfunction has occurred, proteolytic activity of multi-hydrolases is likely to be inhibited, with no ensuing degradation of aberrant proteins detrimental to neuronal survival (McNaught, 2004). Some scholars revealed that chymotrypsin-, trypsin- and post-acidic-like hydrolyzing activities of the proteasome are remarkably impaired in the substantia nigra in autopsy tissue of PD patients (McNaught and Jenner, 2001; McNaught et al., 2006). As far as proteasome inhibitors are concerned, lactacystin (an irreversibleproteasome inhibitor) and MG132 (a reversible proteasome inhibitor) both lead to proteasomal dysfunction, and subsequently, preferential degeneration of DA neurons (Rideout et al., 2005; McNaught et al., 2010; Fan et al., 2016). In this study, we also observed the same damaging effects in lactacystinand MG132-treated PC12 cells. Viability of PC12 cells was reduced aer lactacystin/MG132 administration, which was accompanied by membrane shrinkage and rugosity, and accumulation of alpha-synuclein protein. Altogether, this is indicative of damaged proteasomal function, which is consistent with previous reports in other models (Lindholm et al., 2007; Airavaara et al., 2012; Ren et al., 2013; Mei and Niu, 2015a).

Figure 1 Protective and reversible e ff ects of CDNF on degenerative PC12 cells.
Nonetheless, CDNF has been shown to protect and restore DA neurons induced by 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridinein vivo(Lindholm et al., 2007; Voutilainen et al., 2011; Airavaara et al., 2012; Mei and Niu, 2015b). While the proteasome is responsible for degeneration of abnormal proteins, and plays a major protective role in DA neurons (McNaught et al., 2002; Aguileta et al., 2015). Hence, it is of interest to determine whether CDNF induces neurotrophic e ff ects by modulating proteasome activity. Here, we first confirmed that CDNF exhibits protective and reversible e ff ects on lactacystin- and MG132-induced PC12 cells, with no significant difference found between the groups. Furthermore, alpha-synuclein accumulation, the hallmark of PD, was attenuated by CDNF, similar to the e ff ect of glial cell-derived neurotrophic factor and brain-derived neurotrophic factor (Sathiya et al., 2013; Böttner et al., 2015). Additionally, these previous studies found that CDNF provides protective and reversible e ff ects on endoplasmic reticulum stress (Lindholm and Saarma, 2010; Voutilainen et al., 2015). Based on regulation of alpha-synuclein expression by CDNF, this also illustrates that CDNF may exert neurotrophic e ff ectsviaregulation of proteasome activity. Bedford et al. (2008) con fi rmed that depletion of the 26S proteasome causes neurodegeneration of DA neuronsin vivo. We also found that 26S proteasome activity is upregulated protectively and reversibly by CDNF in the MG132 group. In contrast, only protective upregulation of 26S proteasome activity was observed in the lactacystingroup.is may be because lactacystin achieves irreversible proteasome inhibition, in contrast with the reversible e ff ect of MG132. When the 26S proteasome is inhibited by lactacystin, its function would not be reversed by CDNF, and may be related to translocation of proteolytically cleaved PKCdelta fragments to mitochondria by MG-132 (Sun et al., 2008). This may also explain why we did not observe a reversible CDNF e ff ect aer lactacystin pretreatment. As far as timely and adequate proteasomal processing of abnormal proteins, not only should the number of proteasomes be su ffi cient, but its enzymes are also likely to be essential (Wang et al., 2015).
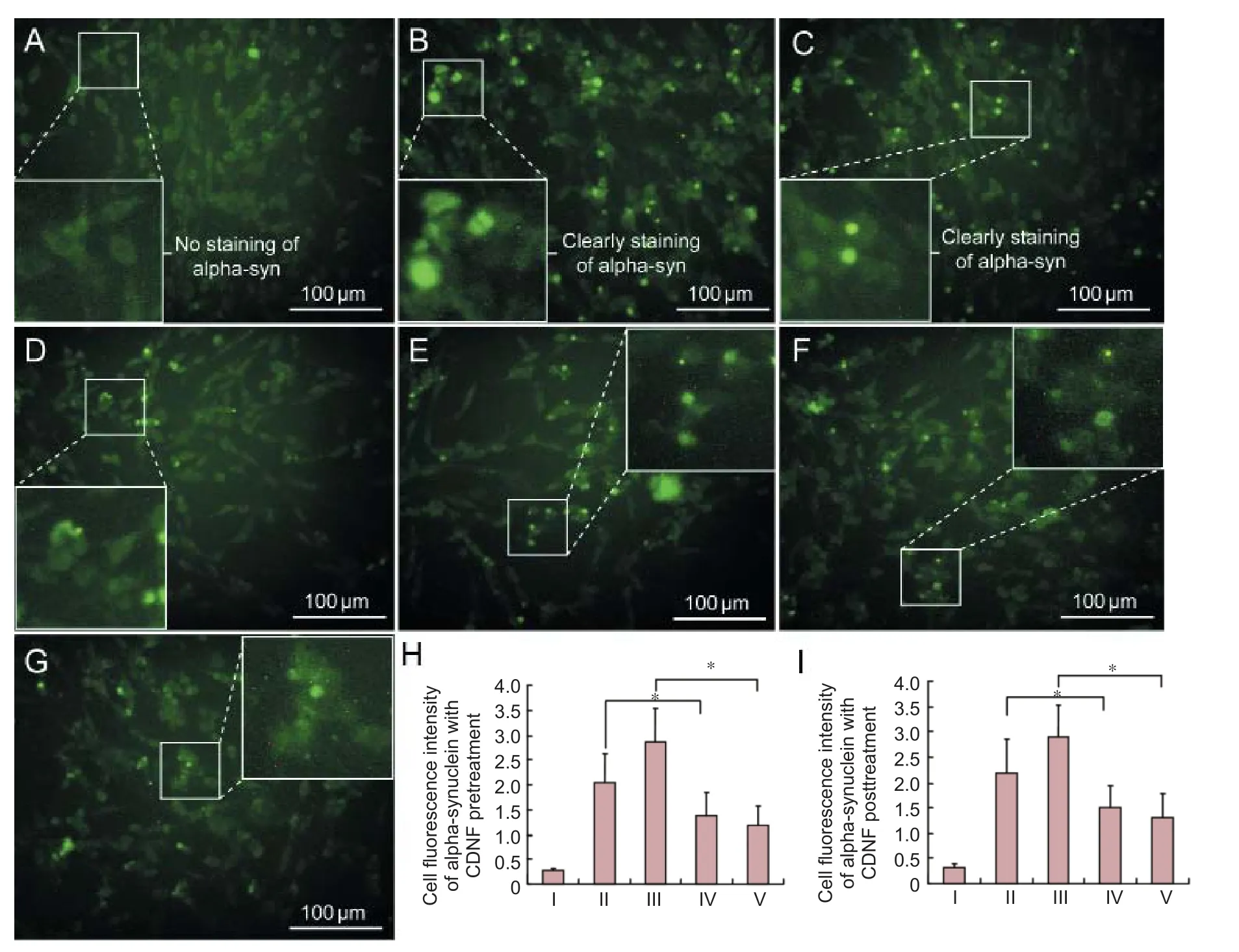
Figure 2 CDNF e ff ects on alpha-synuclein expression in degenerative PC12 cells.
In a previous study, enzymes (glutamyl peptide hydrolase, chymotrypsin, and trypsin) located in the 20S proteasome were visibly reduced in postmortem brain of PD patients (Bukhatwa et al., 2010; Alexopoulou et al., 2016). In this study, we were also interested to examine enzyme activities reflecting 20S proteasomal function. As with glutamyl peptide hydrolase, CDNF played a significant protective role in upregulation in the lactacystin and MG132 groups, and reversible overexpression in the CDNF-lactacystin and CDNF-MG132 groups, with a more pronounced effect in the latter. Regarding the protective and reversible e ff ects on chymotrypsin and trypsin activities in the lactacystin and MG132 groups by CDNF, we found a protective effect on chymotrypsin activity in only MG132-induced PC12 cells. Moreover, no reversible role on chymotrypsin activity was observed between the two groups, although MG132 activity was statistically greater compared with the lactacystin group. More interestingly, no protective or reversible effects were observed on trypsin activity in the lactacystin and MG132 groups, although activity in the MG132 group was statistically greater compared with the lactacystin group. Of the three enzymes located in the 20S proteasome, CNDF exerts protective and reversible modulation on only glutamyl peptide hydrolase, with no statistical e ff ect on the other enzymes. Further research is needed on the underlying mechanism of this process. It is possible that proteasomal dysfunction may not be the sole factor in PD pathogenesis because previousstudies have con fi rmed that PD is also related to endoplasmic reticulum stress and apoptosis (Zhang et al., 2015; Fatkullina et al., 2017). Further, CDNF exerts a neurotrophic e ff ectviamodulation of Bcl-2/Bax and caspase-3 (Mei and Niu, 2014).

Figure 3 E ff ect of CDNF on 26S proteasome expression.

Figure 4 E ff ect of CDNF on proteasome hydrolase.
Based on our results, we have shown that CDNF plays neuroprotective and reversible roles in PC12 cells aer proteasome inhibition, mainlyviaregulation of the 26S proteasome and glutamyl peptide hydrolase activity. For lactacystin, reversible proteasomal inhibition of MG132 appears to be reflected mainly in reversible inhibition of the 26S proteasome and glutamyl peptide hydrolase enzyme. While for reversible proteasomal inhibition of MG132, protection of the 26S proteasome and its enzymes by CDNF was greater in the MG132 group compared with the lactacystin group. Certainly, PC12 cells are a widely accepted cell model of PD, and not just DA neurons. Nonetheless, further studies are needed to identify the neurotrophic e ff ect of CDNF in DA neurons. Overall, we have preliminarily demonstrated that CDNF exerts neurotrophic e ff ectsviaregulation of proteasomal activities, although the mechanism needs further research.
Acknowledgments:Xiao-hua Wang from the First Affiliated Hospital of Anhui Medical University co-contributed to statistical analysis in this work.
Author contributions:JMM and CSN designed this study. JMM analyzed and interpreted the data, wrote the paper, cooperated statistical analysis, and performed experiments in Anhui Key Laboratory of Brain Function and Brain Disease in China. CSN revised the paper. Both of these two authors approved the fi nal version of the paper.
Con fl icts of interest:None declared.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Aguileta MA, Korac J, Durcan TM, Trempe JF, Haber M, Gehring K, Elsasser S, Waidmann O, Fon EA, Husnjak K (2015)e E3 ubiquitin ligase parkin is recruited to the 26 S proteasome via the proteasomalubiquitin receptor Rpn13. J Biol Chem 290:7492-7505.
Airavaara M, Harvey BK, Voutilainen MH, Shen H, Chou J, Lindholm P, Lindahl M, Tuominen RK, Saarma M, Hoffer B, Wang Y (2012) CDNF protects the nigrostriatal dopamine system and promotes recovery aer MPTP treatment in mice. Cell Transplant 21:1213-1223.
Alexopoulou Z, Lang J, Perrett RM, Elschami M, Hurry ME, Kim HT, Mazaraki D, Szabo A, Kessler BM, Goldberg AL, Ansorge O, Fulga TA, Tofaris GK (2016) Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. Proc Natl Acad Sci U S A 113:E4688-4697.
Anusha C, Sumathi T, Joseph LD (2017) Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroin fl ammation and oxidative stress mediated apoptosis. Chem Biol Interact 269:67-79.
Bäck S, Peränen J, Galli E, Pulkkila P, Lonka-Nevalaita L, Tamminen T, Voutilainen MH, Raasmaja A, Saarma M, Männistö PT, Tuominen RK (2013) Gene therapy with AAV2-CDNF provides functional bene fi ts in a rat model of Parkinson’s disease. Brain Behav 3:75-88.
Bedford L, Hay D, Devoy A, Paine S, Powe DG, Seth R, Gray T, Topham I, Fone K, Rezvani N, Mee M, Soane T, Lay fi eld R, Sheppard PW, Ebendal T, Usoskin D, Lowe J, Mayer RJ (2008) Depletion of 26 s proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci 28:8189-8198.
Bentea E, Van der Perren A, Van Lie ff eringe J, El Arfani A, Albertini G, Demuyser T, Merckx E, Michotte Y, Smolders I, Baekelandt V, Massie A (2015) Nigral proteasome inhibition in mice leads to motor and non-motor de fi cits and increased expression of Ser129 phosphorylated α-synuclein. Front Behav Neurosci 9:68.
Böttner M, Fricke T, Müller M, Barrenschee M, Deuschl G, Schneider SA, Egberts JH, Becker T, Fritscher-Ravens A, Ellrichmann M, Schulz-Schae ff er WJ, Wedel T (2015) Alpha-synuclein is associated with the synaptic vesicle apparatus in the human and rat enteric nervous system. Brain Res 1614:51-59.
Bukhatwa S, Zeng BY, Rose S (2010) A comparison of changes in proteasomal subunit expression in the substantia nigra in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. Brain Res 1326:174-183.
Chu TT, Gao N1, Li QQ, Chen PG, Yang XF, Chen YX, Zhao YF, Li YM (2016) Speci fi c knockdown of endogenous tau protein by peptide-directed ubiquitin-proteasome degradation. Cell Chem Biol 23:453-461.
Fan T, Huang Z, Chen L, Wang W, Zhang B, Xu Y, Pan S, Mao Z, Hu H, Geng Q (2016) Associations between autophagy, the ubiquitin-proteasome system and endoplasmic reticulum stress in hypoxia-deoxygenation or ischemia-reperfusion. Eur J Pharmacol 791:157-167.
Fatkullina LD, Molochkina EM, Kozachenko AI, Nagler LG, Burlakova EB (2017) Structural and functional state of erythrocyte membranes in mice at different stages of experimental parkinson’s disease induced by administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Bull Exp Biol Med 162(5):597-601.
Jnobaptiste R, Yabut J, Shashidharan P, Jennert P, Olanow CW (2002) Impairment of the ubiquitin-proteasome system causes dopaminergic cell death and inclusion body formation in ventral mesencephalic cultures. J Neurochem 81:301-306.
Lindholm P, Saarma M (2010) Novel CDNF/MANF family of neurotrophic factors. Dev Neurobiol 70:360-371.
Lindholm P, Voutilainen MH, Laurén J, Peränen J, Leppänen VM, Andressoo JO, Lindahl M, Janhunen S, Kalkkinen N, Timmusk T, Tuominen RK, Saarma M (2007) Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature 448:73-77.
McNaught KS (2004) Proteolytic dysfunction in neurodegenerative disorders. Int Rev Neurobiol 62:95-119.
McNaught KS, Björklund LM, Belizaire R (2002) Proteasome inhibition causes nigral degeneration with inclusion bodies in rats. Neuroreport 13:1437-1441.
McNaught KS, Jenner P (2001) Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci Lett 297:191-194.
McNaught KS, Perl DP, Brownell AL, Olanow CW (2004) Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Ann Neurol 56:149-162.
McNaught KS, Jackson T, JnoBaptiste R (2006) Proteasomal dysfunction in sporadic Parkinson’s disease. Neurology 66:S37-49.
McNaught KS, Jnobaptiste R, Jackson T, Jengelley TA (2010)e pattern of neuronal loss and survival may re fl ect di ff erential expression of proteasome activators in Parkinson’s disease. Synapse 64:241-250.
Mei J, Niu C (2015a) Protective and reversal e ff ects of conserved dopamine neurotrophic factor on PC12 cells following 6-hydroxydopamine administration. Mol Med Rep 12:297-302.
Mei J, Niu C (2015b) E ff ects of engineered conserved dopamine neurotrophic factor-expressing bone marrow stromal cells on dopaminergic neurons following 6-OHDA administrations. Mol Med Rep 11:1207-1213.
Mei JM, Niu CS (2014) E ff ects of CDNF on 6-OHDA-induced apoptosis in PC12 cells via modulation of Bcl-2/Bax and caspase-3 activation. Neurol Sci 35:1275-1280.
Nair VD, McNaught KS, González-Maeso J, Sealfon SC, Olanow CW (2006) p53 mediates nontranscriptional cell death in dopaminergic cells in response to proteasome inhibition. J Biol Chem 281:39550-39560.
Niu C, Mei J, Pan Q, Fu X (2009) Nigral degeneration with inclusion body formation and behavioral changes in rats aer proteasomal inhibition. Stereotact Funct Neurosurg 87:69-81.
Ren X, Zhang T, Gong X, Hu G, Ding W, Wang X (2013) AAV2-mediated striatum delivery of human CDNF prevents the deterioration of midbrain dopamine neurons in a 6-hydroxydopamine induced parkinsonian rat model. Exp Neurol 248:148-156.
Rideout HJ, Lang-Rollin IC, Savalle M (2005) Dopaminergic neurons in rat ventral midbrain cultures undergo selective apoptosis and form inclusions, but do not up-regulate iHSP70, following proteasomal inhibition. J Neurochem 93:1304-1313.
Sathiya S, Ranju V, Kalaivani P, Priya RJ, Sumathy H, Sunil AG, Babu CS (2013) Telmisartan attenuates MPTP induced dopaminergic degeneration and motor dysfunction through regulation of α-synuclein and neurotrophic factors (BDNF and GDNF) expression in C57BL/6J mice. Neuropharmacology 73:98-110.
Sun F, Kanthasamy A, Song C, Yang Y, Anantharam V, Kanthasamy AG (2008) Proteasome inhibitor-induced apoptosis is mediated by positive feedback ampli fi cation of PKCdelta proteolytic activation and mitochondrial translocation. J Cell Mol Med 12:2467-2481.
Voutilainen MH, Bäck S, Peränen J, Lindholm P, Raasmaja A, Männistö PT, Saarma M, Tuominen RK (2011) Chronic infusion of CDNF prevents 6-OHDA-induced de fi cits in a rat model of Parkinson’s disease. Exp Neurol 228:99-108.
Voutilainen MH, Arumäe U, Airavaara M, Saarma M (2015) Therapeutic potential of the endoplasmic reticulum located and secreted CDNF/MANF family of neurotrophic factors in Parkinson’s disease. FEBS Lett 589:3739-3748.
Wang R, Zhao J, Zhang J, Liu W, Zhao M, Li J, Lv J, Li Y (2015) E ff ect of lysosomal and ubiquitin-proteasome system dysfunction on the abnormal aggregation of α-synuclein in PC12 cells. Exper Med 9:2088-2094.
Xie W, Li X, Li C, Zhu W, Jankovic J, Le W (2010) Proteasome inhibition modeling nigral neuron degeneration in Parkinson’s disease. J Neurochem 115:188-199.
Zhang GF, Zhang Y, Zhao G (2015) Crocin protects PC12 cells against MPP(+)-induced injury through inhibition of mitochondrial dysfunction and ER stress. Neurochem Int 89:101-110.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
10.4103/1673-5374.211195
Accepted: 2017-05-06
*Correspondence to: Chao-shi Niu, Ph.D., doctorstar@126.com.
- 中国神经再生研究(英文版)的其它文章
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Recovery of sympathetic nerve function after lumbar sympathectomy is slower in the hind limbs than in the torso
- Short-term observations of the regenerative potential of injured proximal sensory nerves crossed with distal motor nerves
- Aldehyde dehydrogenase 2 overexpression inhibits neuronal apoptosis after spinal cord ischemia/ reperfusion injury
- Long-term acupuncture treatment has a multitargeting regulation on multiple brain regions in rats with Alzheimer’s disease: a positron emission tomography study
- E ff ect of glycosides of Cistanche on the expression of mitochondrial precursor protein and keratin type II cytoskeletal 6A in a rat model of vascular dementia

