黄颡鱼性别决定和分化相关基因的鉴定及其在性腺中的表达分析
谢彬月+熊舒婷+梅洁

摘要:为探索黄颡鱼(Pelteobagrus fulvidraco)性別决定和分化的分子机制,在黄颡鱼性腺转录组数据中筛选到13个相关基因。表达谱分析表明,雄性相关基因中,piwi-1和amhr2在XY雄鱼和YY超雄鱼中表达量明显高于XX雌鱼,amh在XY雄鱼中表达量高于XX雌鱼和YY超雄鱼。雌性相关基因中,cyp19a和foxl2在XY雄鱼和YY超雄鱼中几乎不表达,但在XX雌鱼中有一定表达。amh和amhr2的进一步研究表明,amh和amhr2在性腺中均有高表达量。XY雄鱼腹腔注射EE2后,amh和amhr2呈现先上升随后下调的趋势。XX雌鱼腹腔注射MT后,48 h和72 h时amh的表达量均低于正常水平,120 h恢复正常;amhr2在MT处理48 h后表达显著下调,72 h和120 h时约为正常水平的3/5。
关键词:黄颡鱼(Pelteobagrus fulvidraco);基因表达;性别决定和分化;激素处理
中图分类号:Q953+.3 文献标识码:A 文章编号:0439-8114(2017)12-2362-06
DOI:10.14088/j.cnki.issn0439-8114.2017.12.041
Identification and Expression of Genes Related to Sex Determination and Differentiation in Pelteobagrus fulvidraco
XIE Bin-yue, XIONG Shu-ting, MEI Jie
(College of Fisheries, Huazhong Agricultural University/Key Laboratory of Freshwater Animal Breeding,
Ministry of Agriculture,Wuhan 430070,China)
Abstract:To investigate the molecular mechanism of sex determination and differentiationin Yellow Catfish(Pelteobagrus fulvidraco),we screened out 13 related genes from the transcriptome data of gonads. The expression profile of these 13 genes indicated that the male sex-related genes such as piwi-1 andamhr2had higher expression in XY and YY testsis than in XX ovary, and the expression of amhin XY testis was higher than in XX ovary and YY testis. There were almost no expression of cyp19a and foxl2 in XY and YY testis, whereas their expressions in XX ovary were detected. We chose amh and amhr2genes for further research.RT-PCR results indicated that amh and amhr2 were highly expressed in the gonad among the hypothalamus-pituitary-gonad axis and liver. For the hormone treatment experiment, healthy XX female individuals were injected with 17α-methyltestosterone (MT) and XY male individuals with 17α-ethinylestradiol(EE2). EE2 treatment resulted in upregulation of amh and amhr2 at first and subsequent downregulation in males. After MT treatment in females,the relative expression of amhin ovary was lower than normal level at 48 hours and 72 hours post treatment(hpt) but return to normal at 120 hpt. The amhr2 expression was significantly reduced at 48 hpt and was about 3/5 of the control at 72 and 120 h.
Key words: Pelteobagrus fulvidraco; gene expression; sex determination and differentiation; hormone treatment
硬骨鱼类的性别决定与性腺分化受遗传因素和外界环境因素共同作用的影响。大多数鱼类性别主要决定于基因型,其性腺分化和发育过程受到内在因素和外部环境(激素、温度、pH、密度)的调控[1],并通过下丘脑-垂体-性腺(Hypothalamus-pituitary-gonads axis,HPG轴)来实现[2]。黄颡鱼(Pelteobagrus fulvidraco)是中国一种重要的淡水经济鱼类,雌雄生长差异显著,在相同的生长条件下,雄性黄颡鱼生长速度明显高于雌性。刘汉勤等[3]、Wang等[4]采用激素性逆转结合雌核发育技术,从XY雌鱼产生可育YY超雄黄颡鱼,大大提高了后代的雄性比例。
鱼类中首次鉴定到的性别决定基因是日本青鳉Y染色体连锁的Dmy/Dmrt1bY基因[5]。隨后,吕宋青鳉(Oryziasluzonensis)中的gsdf、恒河青鳉(Oryziasdancena)中的sox3、河豚(Takifugurubripes)中的amhr2、牙汉鱼(Odontestheshatcheri)和罗非鱼中的amhy以及虹鳟中的sdY也相继被鉴定为性别决定基因。研究证实,Dmy、sox3、amhr2、sdY和amhy基因的突变或敲除都导致了XY型雌鱼,sox3、SdY、gsdf和amhy的转基因过表达则产生XX型雄鱼,说明它们是雄性决定基因,在雄性决定过程中起着关键作用;而foxl2基因的敲除导致了XX型雄鱼,说明foxl2在雌性决定过程中起着关键作用[6-11]。vasa的功能性缺失可以导致雌性不孕或者雄性不育,但在不同物种中有不同表型,如斑马鱼中vasa基因突变可以导致雄性因不能形成生殖细胞而不育[12],而果蝇中vasa基因沉默会导致雌性个体因卵子发生严重缺陷而不育[13]。
前期研究中,对1龄黄颡鱼中的XX雌鱼、XY雄鱼和YY超雄鱼的性腺进行比较转录组分析,其中XY精巢和YY精巢的比较结果已经发表,发现一些与精巢发育和精子生成相关的基因[14]。对未发表的XX卵巢转录组和已经发表的XY精巢转录组进行比较分析,发现了一些与鱼类性别决定和分化相关基因。参照已有研究中总结的XX/XY性别决定系统鱼类的性别决定基因[1],农业部淡水生物繁育重点实验室黄颡鱼转录组数据鉴别出来的基因中筛选到13个可能与性别决定和分化相关的基因,并使用转录组测得的RPKM值制作了XX雌鱼、XY雄鱼、YY超雄鱼中各基因的表达谱作进一步分析。
抗缪勒氏管激素(Anti-mullerian hormone,Amh)为糖蛋白,属于转化生长因子-β(Transforming growth factor-β,TGF-β)细胞因子家族[15],在精巢和卵巢中都有表达[16,17],在哺乳动物中amh诱导雌性生殖原基缪勒氏管退化,使个体向雄性方向发育,抗缪勒氏管激素特异性受体Ⅱ(AMH specific type Ⅱ receptor,Amhr2)为其特异性受体。最初认为amh只能特异性作用于性腺和生殖器官上,并被称为“具有多种功能的性激素”[18],然而近些年研究人员发现amh和amhr2也存在于小鼠的脑中,特别是下丘脑、垂体中[19-21],并通过HPG轴参与调节雌雄性腺类固醇激素的生成、胸腺与前列腺生长及卵泡增生等多种生物学功能[21]。因此,本试验检测了amh和amhr2在黄颡鱼HPG和肝脏中的表达量,并选取17α-乙炔雌二醇(ethinylestradiol,EE2)和17α-甲基睾酮(17α-methyltestosterone,MT)两种类固醇激素分别处理了雄性和雌性黄颡鱼,检测其对amh和amhr2基因表达的影响。
1 材料与方法
1.1 试验动物
试验鱼采集于湖北省荆州市的养殖基地,选择1龄性成熟、大小均一的健康黄颡鱼个体于实验室水缸中暂养1周,给水体不间断充气,水温维持在26 ℃。试验鱼的遗传性别鉴定采用Dan等[22]开发的性别特异分子标记。所有的试验操作均按照华中农业大学动物保护和使用委员会的要求进行。
1.2 黄颡鱼转录组数据的分析
将已发表文章中的1龄XY黄颡鱼精巢转录组数据[14]和同龄同批次测的XX卵巢转录组数据进行比较分析。对于所有未处理的读长,按照已报道的方法[23]使用LUCY和Seq-Clean软件剔除低质量的碱基和接头引物。使用Newbler software package(Roche公司)进行序列组装。利用本地BLAST软件搜索NCBI非冗余核苷酸数据库(Nt)、STRING数据库、GENE数据库进行基因注释,E≤le-5。每条序列按照BLAST匹配中取得的最高比对分指定基因名称。
1.3 激素处理
激素处理采取腹腔注射的方法,从胸鳍后对雄性个体注射17α-乙炔雌二醇(17α-ethinylestradiol,EE2),雌性个体注射17α-甲基睾酮(17α-methyltestosterone,MT)。在注射前,先将EE2和MT溶解到5%乙醇/95%生理盐水混合溶液中,对照组只注射5%乙醇/95%生理盐水混合溶液。Xiong等[24]发现10 μg MT/g注射处理是对内源性性激素产生相对稳定效果的最佳剂量,可以使内源性17β-雌二醇(17β-estradiol,E2)持续下降,内源性11-酮基睾酮(11-ketotestosterone,11-KT)持续增长。10 μg EE2/g是鱼类注射的常用浓度,对黄颡鱼的近缘物种瓦氏黄颡鱼(Pelteobagrus vachelli)具有明显的雌激素效应[25]。本试验中MT和EE2均采用10 μg/g的剂量注射。分别取处理后不同时间的精巢和卵巢组织(每组4或5条鱼)。取样的组织即可放入液氮中冷冻保存,等待提取RNA。
1.4 RNA提取、反转录和荧光定量PCR
使用美国QIAGEN公司的miRNeasy Mini Kit提取总RNA,按照使用说明书进行操作。使用Invitrogen公司的GoldScript cDNA Synthesis Kit对1 μg总RNA进行反转录。引物设计采用Primer 5.0软件。引物经过PCR验证不会产生二聚体,序列也经过测序验证。qRT-PCR在Bio-Rad公司的Bio-Rad PCR system by CFX96 Optics Module CFX96实时荧光定量PCR仪系统上进行,使用SYBR Green I染料[26,27]。qPCR体系为20 μL,包括10 μL 2×SYBR green master mix,1 μL模板,8 μL H2O,引物(10 μmmol/L)各0.5 μL。qPCR程序为95 ℃ 1 min,95 ℃ 15 s,56 ℃ 20 s,72℃ 20 s,77 ℃ 5 s,79 ℃ 5 s,81 ℃ 5 s,溶解曲线65~95 ℃,按照0.5 ℃/s的速度升温,每个温度持续0.5 s。无模板反应作为阴性对照,每个反应的扩增特异性使用CFX管理软件(Bio-Rad)中的溶解曲线进行分析。前期检测性激素处理后6个管家基因(β-actin、rp17、GAPDH、e1fa、b2m及18S rRNA)的转录稳定性,经过geNorm软件分析,发现β-actin(M=1.039<1.5)是最稳定的[25],因此选择β-actin作为内参基因。每个试验重复3次。
1.5 数据分析
参照Wei等[28]的方法,将基因表达谱中的RPKM值划分为7个等级,并用不同的颜色予以表示。相对表达量由qRT-PCR分析得出。使用SPSS 20.0软件(SPSS,USA)进行统计学分析,3个独立的试验数据用mean±SD表示。对照组和处理组的显著差异使用单因素方差分析t值检验,P<0.05被视为统计学显著性差异。
2 结果与分析
2.1 在黄颡鱼转录组数据中筛选并确认与性别决定和分化相关的基因
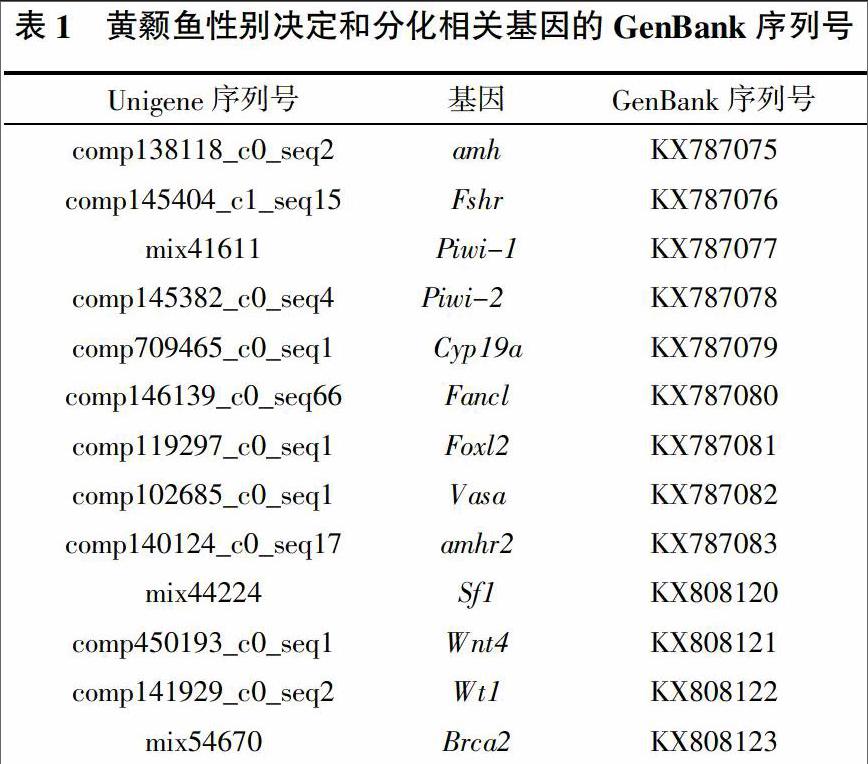
在黄颡鱼转录组数据中筛选到13个可能与性别决定和性腺发育相关的基因,经BlAST比对分析确认以下Unigene为正确拼接序列并提交到GenBank获得序列号(表1)。
2.2 黄颡鱼性别决定和分化相关基因的表达谱
黄颡鱼性别决定和分化相关基因在转录组中的表达水平分析如图1,雄性相关基因中,Piwi-1在3种鱼中表达量均较高,XY雄鱼和YY超雄鱼中表达量明显高于XX雌鱼。amh和amhr2在3种鱼中表达量均较低,但是amh在XY雄鱼中表达量各为XX雌鱼和YY超雄鱼的5倍左右,amhr2在XX雌鱼中几乎不表达,在XY雄鱼和YY超雄鱼中的表达量明显高于XX雌鱼。
雌性相关基因中,cyp19a和foxl在XY雄鱼和YY超雄鱼中几乎不表达,但在XX雌鱼中有低表达。vasa在3种鱼中表达量均较高,其在YY超雄鱼中表达量最高,XY雄鱼中次之,各约为XX雌鱼中的5倍和4倍。wnt4在XX雌鱼和XY雄鱼中几乎不表达,但在YY超雄鱼中低表达。在XY雄鱼中,cyp19a几乎不表达。
2.3 amh和amhr2在HPG轴和肝脏中的相对表达量
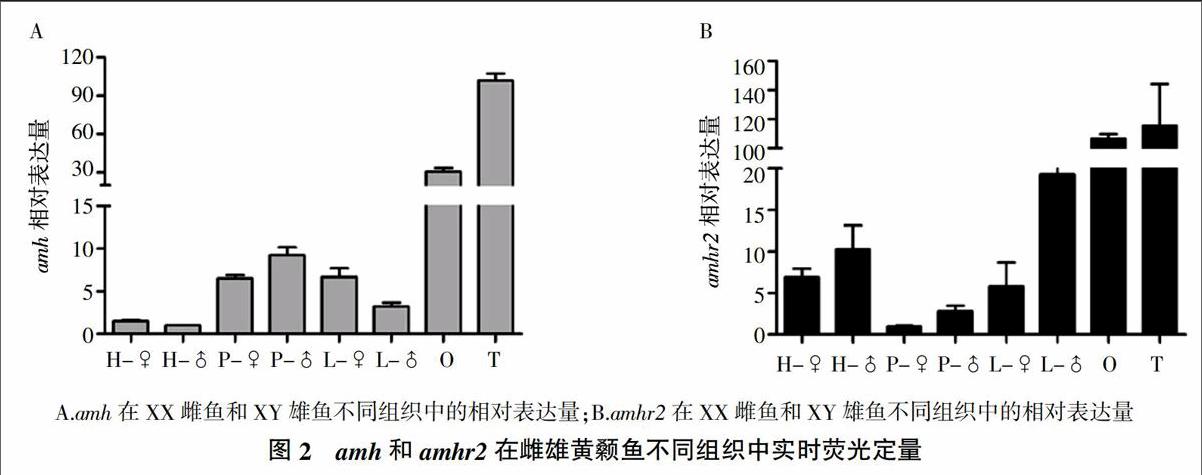
如图2中的定量PCR结果所示,amh和amhr2在1龄雌、雄黄颡鱼性腺中表达量最高,并且都在下丘脑、垂体和肝脏中有表达。amh在雄鱼性腺和垂体中的表达量显著高于雌鱼,而肝脏雌鱼amh水平高于雄鱼。amhr2在雌、雄黄颡鱼性腺中表达差异不显著,而在雄鱼下丘脑、垂体和肝脏中的表达水平显著高于雌性。
2.4 性激素对amh和amhr2在性腺中表达的影响
图3A和3B表明,XY雄魚腹腔注射EE2处理后,amh在处理后48 h之前呈现上升的趋势随后显著下调并低于正常水平;而amhr2的表达量在36 h之前呈上升趋势,随后逐渐下调。图3C表明,XX雌鱼腹腔注射MT后,48 h和72 h时amh的表达量均低于正常水平,120 h恢复正常;图3D表明,雌鱼MT处理48 h时amhr2的表达量与正常水平相当,72 h和120 h约为正常水平的3/5。
3 讨论
已有研究证实,dmy、sox3、amhr2、sdY和amhy等基因的突变或敲降都导致了XY型雌鱼,sox3、SdY、gsdf和amhy的转基因过表达则产生XX型雄鱼,说明它们是雄性决定基因,在雄性决定过程中起着关键作用;而foxl2基因的敲除导致了XX型雄鱼,说明foxl2在雌性决定过程中起着关键作用[6-11]。本研究中,与性别决定相关基因的表达谱分析显示,amh、amhr2和piwi-1可能在雄性黄颡鱼性腺发育过程中发挥重要作用,cyp19a、foxl可能在雌性性腺发育中发挥重要作用。
vasa基因最先在果蝇中发现,在果蝇母源性基因的筛选过程中首次发现其在卵母细胞发育方面的重要作用[13]。已有研究表明,vasa基因在不同物种生殖系中有不同的作用,斑马鱼中雄性个体vasa基因缺失会导致精巢发育缺陷而不育[12];果蝇中vasa基因沉默会导致雌性个体因卵子发生严重缺陷而不育[13]。本研究中vasa在雄鱼中的高表达量暗示其在黄颡鱼雄性生殖发育中起着重要的功能。
芳香化酶在雄激素向雌激素的转变过程中必不可少,有研究显示amh和芳香化酶基因之间存在负相关性,如在斑马鱼[29]、虹鳟[30]中amh在精巢中的高表达可以抑制芳香化酶的释放,从而抑制雄激素向雌激素转变的进程。但也有研究表明amh和芳香化酶基因的表达各不相关,如在尼罗罗非鱼(Oreochromis niloticus)[31]中。外源性类固醇激素作为一种有效的合成激素,被吸收到鱼类身体里,并且可能扰乱初始的体内稳态。据报道,雌激素在斑马鱼、日本比目鱼、银汉鱼(Atherina bleekeri)、黑头软口鲦(Pimephales promelas)、黑鲷(Sparus macrocephlus)5种鱼中抑制amh的表达。在黑头软口鲦中,雄鱼使用10 ng EE2/L曝露处理后引起amh表达下降;在斑马鱼幼鱼中EE2处理导致雌性化,并引起amh表达下降;而在青鳉和红树林鳉鱼(Kryptolebias marmoratus)2种鱼中对amh的表达没有作用[32]。但在本试验中,EE2腹腔注射导致雄性黄颡鱼精巢amh表达量上升,推测可能与处理方式和注射剂量有关,即暴露处理使小剂量的EE2进入到斑马鱼体内,可以抑制amh的表达;而腹腔注射使大剂量的EE2进入到斑马鱼体内,并且直接作用于精巢,故而引起amh的表达应激性升高。已有研究表明,出生后amh的水平受到促卵泡激素(follicle-stimulating hormone,Fsh)和雄激素的调节,在雄性中Fsh促进amh的分泌,但是雄激素抑制amh的表达[33,34]。EE2对下丘脑和垂体有正、负反馈作用,推测在雄鱼中小剂量的EE2可以抑制垂体合成和释放Fsh,从而降低amh的表达量;大剂量的EE2可以促进垂体合成和释放Fsh,从而提高amh的表达量。
参考文献:
[1] 梅 洁,桂建芳.鱼类性别异形和性别决定的遗传基础及其生物技术操控[J].中国科学―生命科学,2014,44(12):1198-1212.
[2] 桂建芳,朱作言.水产动物重要经济性状的分子基础及其遗传改良[J].科学通报,2012,59(19):1719-1729.
[3] 劉汉勤,崔书勤,侯昌春,等.从XY雌鱼雌核发育产生YY超雄黄颡鱼[J].水生生物学报,2007,31(5):718-725.
[4] WANG D,MAO H L,CHEN H X,et al. Isolation of Y-and X-linked SCAR markers in yellow catfish and application in the production of all-male populations[J].Animal Genetics,2009, 40(6):978-981.
[5] MATSUDA M,NAGAHAMA Y,SHINOMIYA A,et al.DMY is a Y-specific DM-domain gene required for male development in the medaka fish[J].Nature,2002,417(6888):559-563.
[6] MYOSHO T,OTAKE H,MASUYAMA H,et al. Tracing the emergence of a novel sex-determining gene in medaka,Oryzias luzonensis[J].Genetics,2012,191(1):163-170.
[7] TAKEHANA Y,MATSUDA M,MYOSHO T,et al. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena[J].Nature communications,2014,5:4157.
[8] KAMIYA T,KAI W,TASUMI S,et al. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes(fugu)[J].PLoS Genet,2012,8(7):e1002798.
[9] HATTORI R S,MURAI Y,OURA M,et al. A Y-linked anti-Müllerian hormone duplication takes over a critical role in sex determination[J]. Proceedings of the National Academy of Sciences,2012,109(8):2955-2959.
[10] YANO A,GUYOMARD R,NICOL B,et al. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss[J].Current Biology,2012,22(15):1423-1428.
[11] LI M H,YANG H H,LI M R,et al. Antagonistic roles of Dmrt1 and Foxl2 in sex differentiation via estrogen production in tilapia as demonstrated by TALENs[J].Endocrinology,2013, 154(12):4814-4825.
[12] HARTUNG O,FORBES M M,MARLOW F L. Zebrafish vasa is required for germ-cell differentiation and maintenance[J]. Molecular Reproduction and Development,2014,81(10):946-961.
[13] STYHLER S,NAKAMURA A,SWAN A,et al. Vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development [J]. Development,1998,125(9):1569-1578.
[14] WU J,XIONG S,JING J,et al. Comparative transcriptome analysis of differentially expressed genes and signaling pathways between XY and YY testis in yellow catfish[J].PLoS One,2015,10(8):e0134626.
[15] CATE R L,MATTALIANO R J,HESSION C,et al. Isolation of the bovine and human genes for Mullerian inhibiting substance and expression of the human gene in animal cells[J]. Cell,1986,45(5):685-698.
[16] TEIXEIRA J,MAHESWARAN S,DONAHOE P K. Mullerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications[J].Endocrine Reviews,2001,22(5):657-674.
[17] DURLINGER A L,VISSER J A,THEMMEN A P. Regulation of ovarian function: The role of anti-Mullerian hormone[J]. Reproduction,2002,124(5):601-609.
[18] LEE M M, DONAHOE P K. Mullerian inhibiting substance: A gonadal hormone with multiple functions[J]. Endocrine Reviews,1993,14(2):152-164.
[19] WANG P Y,KOISHI K,MCGEACHIE A B,et al. Mullerian inhibiting substance acts as a motor neuron survival factor in vitro[J]. Proceedings of the National Academy of Sciences of the United States of America,2005,102(45):16421-16425.
[20] LEBEURRIER N,LAUNAY S,MACREZR,et al. Anti-Mullerian-hormone-dependent regulation of the brain serine-protease inhibitor neuroserpin[J].Journal of Cell Science,2008,121(20):3357-3365.
[21] B?魪D?魪CARRATS G Y,?譫NEILL F H,NORWITZ E R,et al. Regulation of gonadotropin gene expression by Müllerian inhibiting substance[J]. Proceedings of the National Academy of Sciences,2003,100(16):9348-9353.
[22] DAN C,MEI J,WANG D,et al. Genetic differentiation and efficient sex-specific marker development of a pair of Y-and X-linked markers in yellow catfish[J]. International Journal of Biological Sciences,2013,9(10):1043-1049.
[23] LIAO X,CHENG L,XU P,et al. Transcriptome analysis of crucian carp(Carassius auratus), an important aquaculture and hypoxia-tolerant species[J]. PloS one,2013,8(4):e62308.
[24] XIONG S,JING J,WU J,et al. Characterization and sexual dimorphic expression of Cytochrome P450 genes in the hypothalamic-pituitary-gonad axis of yellow catfish[J]. General and comparative endocrinology,2015,216:90-97.
[25] YUN L,ZHIQIANG Z H U,QIN Y,et al. Estrogenic effect of 17 β-estradiol on male bagrid catfish Pelteobagrus vachelli[J].Oceanologia et Limnologia Sinica/Hai Yang Yu Hu Chao, 2009,40(2):195-200.
[26] WANG Y,ZHOU L,LI Z,et al. Apolipoprotein C1 regulates epiboly during gastrulation in zebrafish[J]. Science China Life Sciences,2013,56(11):975-984.
[27] ZHONG J X,ZHOU L,LI Z,et al. Zebrafish Noxa promotes mitosis in early embryonic development and regulates apoptosis in subsequent embryogenesis[J]. Cell Death & Differentiation,2014,21(6):1013-1024.
[28] WEI L,YANG C,TAO W,et al. Genome-wide identification and transcriptome-based expression profiling of the sox gene family in the nile tilapia (Oreochromis niloticus)[J]. International Journal of Molecular Sciences,2016,17(3):270-286.
[29] RODR?魱GUEZ-MAR?魱 A,YAN Y L,BREMILLER R A,et al. Characterization and expression pattern of zebrafish Anti-Mullerian hormone(Amh) relative to sox9a,sox9b, and cyp19a1a, during gonad development[J].Gene Expression Patterns,2005, 5(5):655-667.
[30] VIZZIANO D,BARON D,RANDUINEAU G,et al. Rainbow trout gonadal masculinization induced by inhibition of estrogen synthesis is more physiological than masculinization induced by androgen supplementation[J]. Biology of Reproduction, 2008,78(5):939-946.
[31] POONLAPHDECHA S,PEPEY E,CANONNE M,et al. Temperature induced-masculinisation in the Nile tilapia causes rapid up-regulation of both dmrt1 and amh expressions[J]. General and comparative endocrinology,2013,193:234-242.
[32] PFENNIG F,STANDKE A,GUTZEIT H O. The role of Amh signaling in teleost fish-Multiple functions not restricted to the gonads[J]. General and comparative endocrinology,2015, 223:87-107.
[33] REY R,LUKAS-CROISIER C,LASALA C,et al. AMH/MIS: What we know already about the gene, the protein and its regulation[J].Molecular and Cellular Endocrinology,2003,211(1):21-31.
[34] GRINSPON R P,REY R A. Anti-müllerian hormone and sertoli cell function in paediatric male hypogonadism[J].Hormone Research in Paediatrics,2010,73(2):81-92.

