Three Coordination Complexes Based on an Asymmetric Triazole Derivative Ligand: Syntheses,Structures and Photocatalytic Properties
XU Zhou-QingHE Ya-LingLI Hui-Jun*,ZHANG Pei-LingWANG Yuan*,WANG QiZHONG Run-BinJIA Lei*,
(1College of Chemistry and Chemical Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
(2College of Physics and Electronic Information,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
Three Coordination Complexes Based on an Asymmetric Triazole Derivative Ligand: Syntheses,Structures and Photocatalytic Properties
XU Zhou-Qing1HE Ya-Ling1LI Hui-Jun*,1ZHANG Pei-Ling2WANG Yuan*,1WANG Qi1ZHONG Run-Bin1JIA Lei*,1
(1College of Chemistry and Chemical Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
(2College of Physics and Electronic Information,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
Three coordination complexes,namely[Cd2(ptp)2(SO4)(H2O)2]n(1),[Zn(ptp)2(H2O)2](2)and[Cd(ptp)2(H2O)2](3)(Hptp=2-(5-(pyridin-3-yl)-1H-1,2,4-triazol-3-yl)pyrazine),have been synthesized and characterized by elemental analyses,infrared spectra and single-crystal X-ray diffraction analyze.In complex 1,corrugated 1D chains constructed by the ptp-and Cd2+were connected by SO42-to form a 3D structure.In isostructural complexes 2 and 3,intermolecular O-H…N hydrogen bonds link the mononuclear molecules into plane superstructure.The photocatalytic experiment result indicates that in the presence of H2O2,the degradation ratios of methylene blue(MB)reach to 79%,81%and 88%,respectively,when complexes 1,2 and 3 act as catalyst. CCDC:1487968,1;1487969,2;1487970,3.
triazole derivative;complex;photocatalytic degradation
Thestudyofcoordinationcomplexeshas attracted great attentions in the last two decades due notonlytotheirtunablestructures,multiple topologies,but to their potential applications in manyareas such as fluorescent,adsorption and catalytic properties[1-4].Anewemergingapplicationof coordination complexes is photocatalysis,and some results have demonstrated that coordination complexes are efficient photocatalysts on the degradation of organic dyes,water splitting,or photoreduction of CO2[5-7].How to achieve inexpensive,stable,efficient, andband-gaptunablephotocatalystsbased coordination complexes is still a big challenge.
Generally,thepropertiesofcoordination complexes are mainly influenced by several factors, including organic linkers,centre metal ions,pH value, the solvent system and temperature[8-11].Because the ligandcouldadoptdifferentconformationsand alternative linking modes in the crystallization,the rational selection of ligand always plays crucial role in theconstructionofthetargetedcoordination complexes.The triazole ligands offer different charge balance requirements,alternative linking modes,and orientation of donor groups which makes them have thepotentialitytofunctionasbuildingunitsof structural topology[12-15].Furthermore,we think that triazole derivatives should be a kind of appropriate ligand to construct novel coordination complexes with unique structure characters and interesting properties.
Motivated by the above-mentioned facts,2-(5-(pyridin-3-yl)-1H-1,2,4-triazol-3-yl)pyrazine(Hptp),an asymmetric triazolate derivative which contains two potentialbidentatechelatingsitesandtwo monodentate ones,may be a good candidate because the multiple N atoms facilitate the construction of coordination complexes.Herein,we employed Hptp andtransitionalmetalsaltstoachievethree coordination complexes[Cd2(ptp)2(SO4)(H2O)2]n(1),[Zn (ptp)2(H2O)2](2)and[Cd(ptp)2(H2O)2](3).
1 Experimental
1.1 Materials and measurements
Allchemicalswerecommerciallypurchased except for Hptp which was synthesized according to theliterature[16].Elementalanalysesforcarbon, hydrogen and nitrogen were performed on a Thermo Science Flash 2 000 element analyzer.FTIR spectra wereobtainedinKBrdisksonaPerkinElmer Spectrum One FTIR spectrophotometer in 4 000~450 cm-1spectral range.Diffuse reflectance data were collectedusingaShimadzuUV-3600 spectrophotometer,and the Kubelka-Munk function was used to estimate the optical band gap.The powderX-raydiffraction(PXRD)studieswere performed with a Bruker AXS D8 Discover instrument (Cu Kα radiation,λ=0.154 184 nm,U=40 kV,I=40 mA,) over the 2θ range of 5°~50°at room temperature.
1.2 Preparations of the complexes 1~3
1.2.1 Preparation of[Cd2(ptp)2(SO4)(H2O)2]n(1)
A mixture of Hptp(0.05 mmol,11.2 mg),CdSO4·8/3H2O(0.10 mmol,25.6 mg),absolute ethanol(5 mL) and H2O(5 mL)was placed in a Teflon-lined stainless steel vessel(25 mL),heated to 160℃for 3 days,and then cooled to room temperature at a rate of 5℃·h-1. Yellow block crystals of 1 were obtained and picked out,washed with distilled water and dried in air. Yield:30.1 mg,75%(based on Hptp).Elemental analysis Calcd.for C22H18N12O6SCd2(%):C32.89,H 2.26,N 20.92;Found(%):C 32.85,H 2.18,N 20.88. IR(KBr,cm-1):3 437,3 004,1 535,1 497,1 463, 1 406,1 144.
1.2.2 Preparation of[Zn(ptp)2(H2O)2](2)
A mixture of Hptp(0.05 mmol,11.2 mg),Zn (NO3)2·6H2O(0.1 mmol,29.7 mg),absolute ethanol(8 mL)and CH3CN(2 mL)was placed in a sealed flask (25 mL),heated to 160℃for 3 days.Yellow block crystals of 2 were obtained and picked out,washed with distilled water and dried in air.Yield:18.6 mg, 68%(based on Hptp).Elemental analysis Calcd.for C22H18N12O2Zn(%):C 48.23,H 3.31,N 30.68;Found (%):C 48.18,H 3.12,N 30.54.IR(KBr,cm-1):3 437, 2 968,1 533,1 494,1 451,1 413,1 144.
1.2.3 Preparation of[Cd(ptp)2(H2O)2](3)
The same prepared procedure as that for 2 was employed for 3 except that using Cd(NO3)2·4H2O instead of Zn(NO3)2·6H2O.Yellow crystals of 3 were obtained and picked out,washed with distilled water and dried in air.Yield:17.8 mg,60%(based on Hptp).Elemental analysis Calcd.for C22H18N12O2Cd (%):C 44.42,H 3.05,N 28.25;Found(%):C 44.38,H2.98,N28.21.IR(KBr,cm-1):3437,2962,1531,1494, 1451,1415,1144.
1.3.1 X-ray crystallography
X-ray single-crystal diffraction analysis of 1~3 was carried out on a Bruker SMART APEXⅡCCD diffractometer equipped with a graphite monochromated Mo Kα radiation(λ=0.071 073 nm)by using φ-ω scan technique at room temperature.The structures were solved via direct methods and successive Fourier difference synthesis(SHELXS-97)[17],and refined by thefull-matrixleast-squaresmethodonF2with anisotropic thermal parameters for all non-H atoms (SHELXL-97).The empirical absorption corrections were applied by the SADABS program[18].The H-atoms ofcarbonwereassignedwithcommonisotropic displacementfactorsandincludedinthefinal refinement by the use of geometrical restraints.H-atoms of water molecules were first located by the Fourier maps and then refined by the riding mode. The crystallographic data for complexes 1~3 are listed in Table 1.Moreover,the selected bond lengths and bond angles are listed in Table 2.
CCDC:1487968,1;1487969,2;1487970,3.
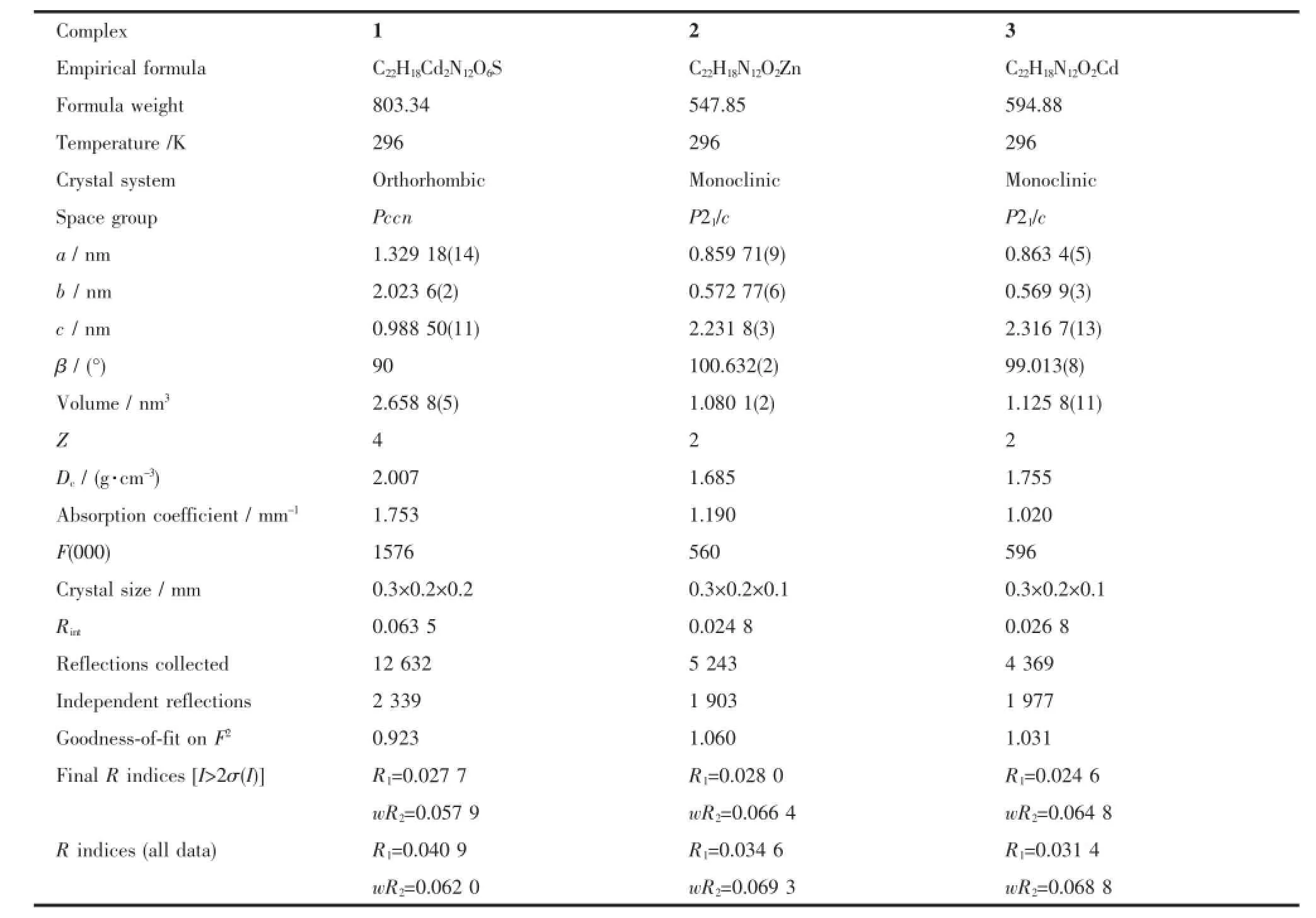
Table 1Crystal data and structural refinement of complexes 1~3
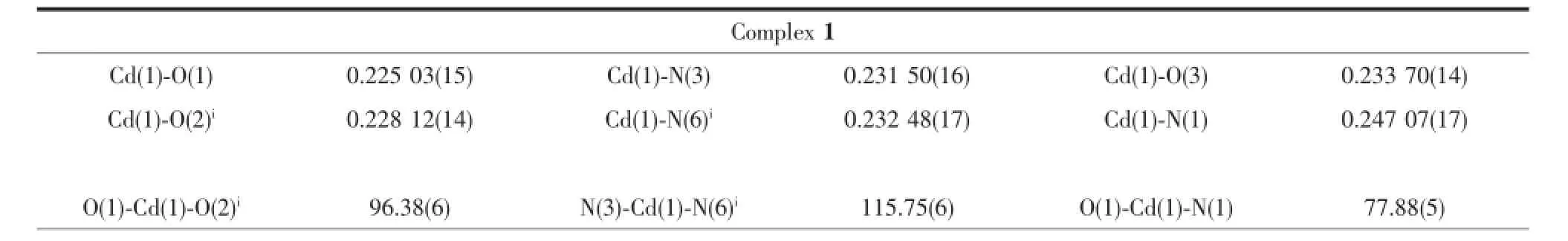
Table 2Selected bond lengths(nm)and angles(°)for complexes 1~3
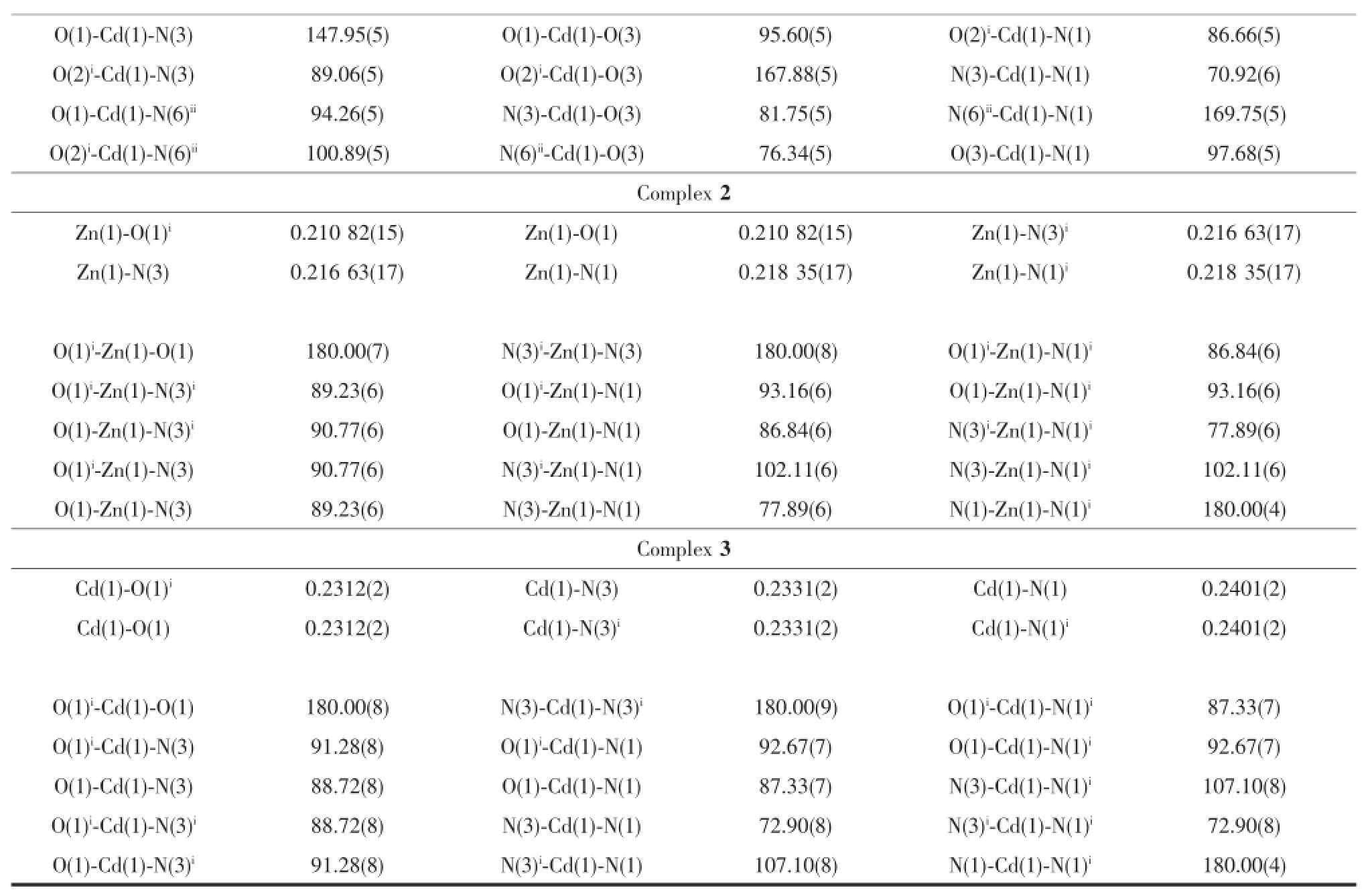
Continued Table 2
1.4 Photocatalytic experiments
To evaluate the photocatalytic activities of these three complexes,the photocatalytic degradation of methylene blue(MB)aqueous solution was performed at ambient temperature.The procedure was as follows: 30 mg of the desolvated samples was dispersed into 100 mL of methylene blue aqueous solution(12.75 mg·L-1),followed by the addition of four drops of hydrogenperoxidesolution(H2O2,30%).The suspensions were magnetically stirred in the dark for over1htoensureadsorptionequilibriumof methylene blue onto the surface of samples.A 300 W xenon arc lamp was used as a light source.An optical filter in the equipment of xenon arc lamp was used to filtering out the UV emission below 400 nm.Then the above solutions were irradiated for 0,20,40,60 and 80 min,and the corresponding reaction solutions were filtered and the absorbance of methylene blue aqueous solutions was then measured by a spectrophotometer. The characteristicpeak(λ=665 nm)forMB was employed to monitor the photocatalytic degradation process.For comparison,the contrast experiment was completed under the same conditions without any cat alyst.
2 Results and discussion
2.1 Crystal structures of complexes 1~3
Single-crystal X-ray measurement reveals that complex 1 crystallizes in the orthorhombic space group Pccn and its asymmetric unit consists of one Cd,one ptp-,one half of SO42-anion and one coordinated water.As shown in Fig.1a,the Cd1 ion is coordinated by three N atoms from two ptp-,two oxygen atoms from two SO42-and one coordinated water molecule,thus creating the distorted octahedron coordination geometry.The ptp-indeed coordinates to two Cdions from a chelating and a bridging site.The Cd-O bond distances are in the range of 0.202 81(14)~0.233 70(14)nm,and the Cd-N bond length is in the range of 0.231 50(16)~0.247 07(17)nm.The Cdions are connected by the ptp-to form an interesting corrugated 1D chain(Fig.1b).We are gratified to note that each SO42-in 1 connects four Cdadopting a μ4,η1η1η1η1-coordination mode.In this way,neighboring chains are connected by SO42-to form the 3D structure of 1(Fig.1c and 1d).
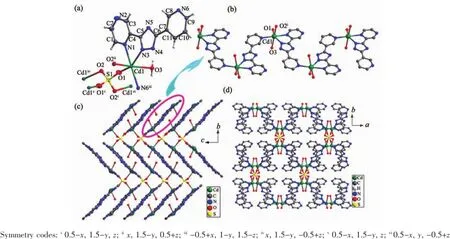
Fig.1View of ORTEP drawing of 1 with 30%thermal ellipsoids(a),zigzag 1D chain(b),3D structure viewed from a axis(c), 3D structure viewed from c axis(d)in 1
Single crystal X-ray diffraction analysis reveals that complexes 2 and 3 are isostructural and crystallize in the monoclinic system,space group P21/c(Table 1). The structure of 2 will be discussed in detail as an example.The asymmetric unit of 2 consists of one half of Zn,one ptp-and one coordinated water molecule. As shown in Fig.2a,the Znpossesses a distorted octahedral coordination environment built by four N atoms from two ptp-and two oxygen atom from coordinatedwatermolecules(Zn-N 0.216 63(17)~ 0.218 35(17)nm,Zn-O 0.210 82(15)nm).
In the crystal of 2,intermolecular O-H…N hydrogen bonds(O1-H1B…N6iand O1-H1B…N4ii, with D…A distances of 0.273 6(2)and 0.277 0(2)nm, D-H…A angles of 162.4°and 167.9°,respectively) link the complex molecules into plane structure, which is similar to the crystal of 3 except that the D…A distances are 0.274 2(3)and 0.277 5(3)nm and D-H…A angles are 160.3°and 173.0°(Fig.2b), respectively.
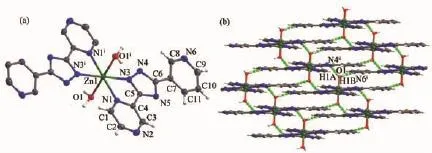
Fig.2View of ORTEP drawing of 2 with 30%thermal ellipsoids(a)and 3D supramolecular architecture connected by hydrogen bonds in 2(b)
2.2 Photocatalysis property
The powder X-ray diffraction(PXRD)patterns for three new complexes are presented in Fig.3,and each experimental pattern is almost same as the simulated pattern,except for a little difference about reflection intensities,which manifests that three new complexes are the purity phase.
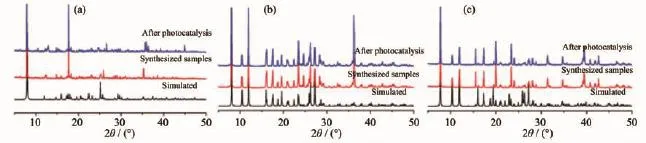
Fig.3Powder XRD patterns for complexes 1(a),2(b)and 3(c)
The band gaps of complexes 1~3 were measured by a solid state ultraviolet-visible(UV-Vis)diffuse reflection measurement method at room temperature. In a plot of K-M function versus energy,the band gap Egis defined as the intersection point among the energyaxisandlineextrapolatedofthelinear portion[19].The K-M function,F=(1-R)2/(2R)(R is the reflectance of an infinitely thick layer at a given wavelength),can be converted from measured diffuse reflectance data.As shown in Fig.4,the Egvalues for complexes 1~3 are 1.85,1.92 and 1.65 eV,respectively. The reflectance spectra show that there exist the optical band gap and semiconductive behaviors in complexes 1~3,and these complexes can be employed as potential semiconductive materials(The Egof a semiconductor is 1~3 eV)[20].
In order to evaluate the photocatalytic effectiveness of the insoluble complexes 1~3,the photocatalytic degradation of ethylene blue(MB)aqueous solution was performed at ambient temperature.Excitingly, complexes 1~3 exhibit good photocatalytic activities in the presence of H2O2under a 300 W xenon lamp irradiation.As can be seen in Fig.5,the characteristic absorption peak of MB(665 nm)were gradually reduced with time increasing from 0 to 60 min. Besides,the changes in the Ct/C0plot of MB solutions versus irradiation time are shown in Fig.6 to clarify thephotocatalyticresults,whereinCtisthe concentration of the MB solutions at t time and C0is the concentration of the MB solutions at the beginning of irridiation.From Fig.6,the degradation ratio of MB reaches17%withoutanyphotocatalyst,whileit increases to 79%,81%and 88%,respectively,when complexes 1,2 and 3 are added to the mixture as catalyst.
Furtherstudiesaboutthestabilityand reproducibility are also carried out shown in Fig.6b, and the results show that the catalytic activities of complexes 1~3 do not exhibit a significant decrease after four runs in the same photocatalytic tests(Table 3),which manifest that the complexes 1~3 as photocatalysts are very stable and possess good reproducibility.
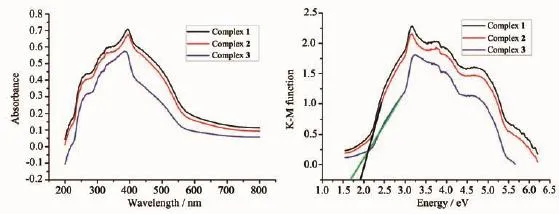
Fig.4(a)UV-Vis absorption spectra of complexes 1~3;(b)Kubelka-Munk-transformed diffuse reflectance of complexes 1~3
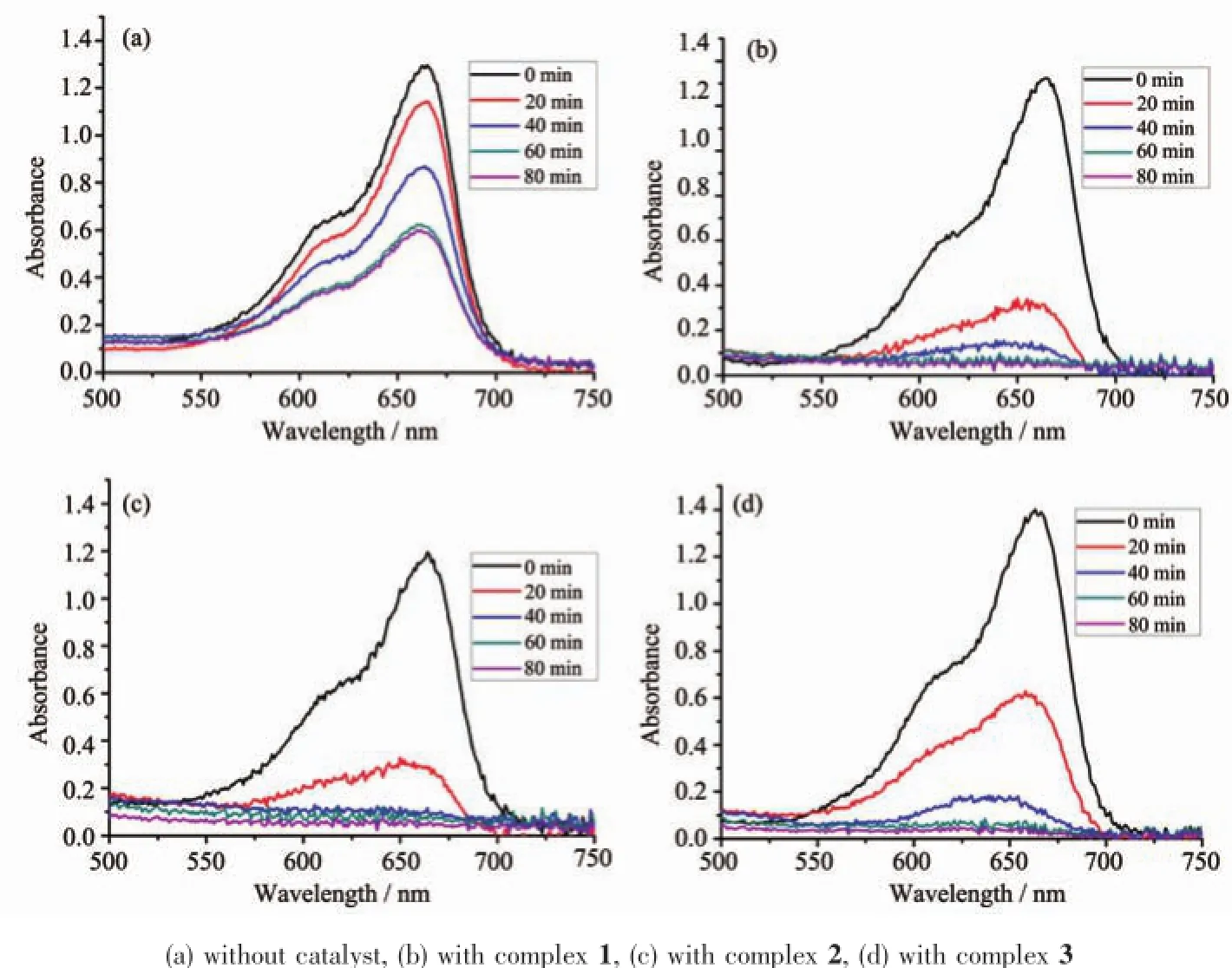
Fig.5Absorption spectra of the solution of MB
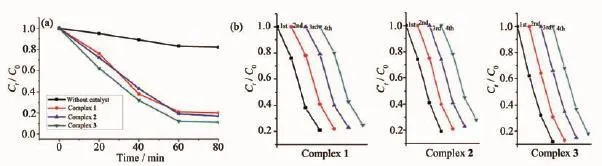
Fig.6(a)Photodegradation of MB with complexes 1~3;(b)Recycling experiments using complexes 1~3 for the photocatalytic degradation of MB solution
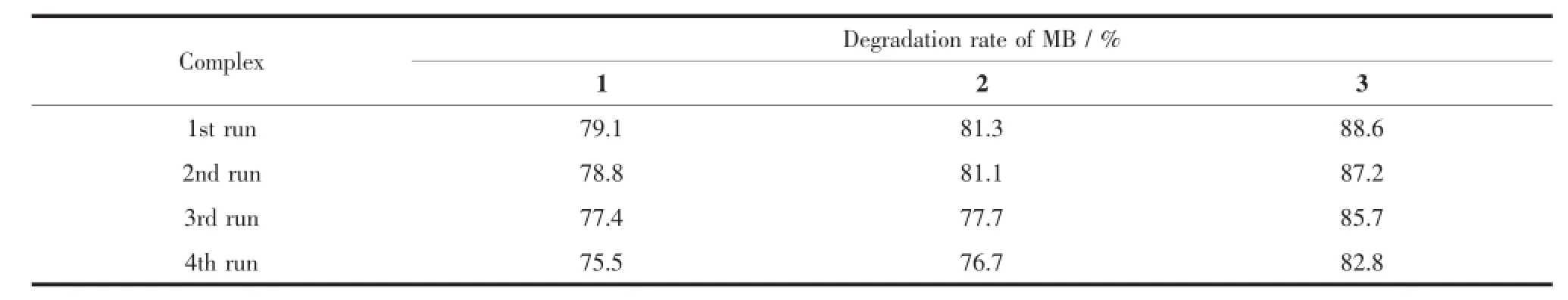
Table 3Recycling experiments using complexes 1~3 for the photocatalytic degradation of MB solution
We know that the efficiency of the photocatalyst isafunctionofthebalancebetweencharge separation,interfacial electron transfer,and charge recombination.In general,a narrow band gap leads to the ease of the charge separation,so the photocatalytic degradation rates of MB should follow the reverse order of band gaps of the complexes.As calculated, the band gaps of complexes 1~3 are 1.85,1.92 and 1.65 eV,respectively.The Egof 1~3 follows the sequence:3<1≈2,and the reverse sequence of band gaps agrees with the degradation rates of MB.In addition,the stabilities of these three complexes are investigated by measuring the powder PXRD patterns after photocatalyticreactions(Fig.3).ThePXRD patterns are consistent with those of the original ones,which confirm that these complexes keep their skeleton well after the photocatalytic process.The photocatalytic research result indicates that complexes 1~3 are goodphotocatalytic catalyst in decomposing MB in the presence of H2O2and may have possible application in decomposing other dyestuff.
3 Conclusions
In summary,an asymmetric triazolate derivative,2-(5-(pyridin-3-yl)-1H-1,2,4-triazol-3-yl)pyrazine(Hptp), was employed to achieve three novel complexes[Cd2(ptp)2(SO4)(H2O)2]n(1),[Zn(ptp)2(H2O)2](2)and[Cd(ptp)2(H2O)2](3).The structures of these complexes were characterizedbysingle-crystalX-raydiffraction, powderX-raydiffraction,elementalanalysesand infrared spectra.The photocatalytic experiment result indicates that complexes 1~3 are good candidates as photocatalysts in decomposing MB in the presence of H2O2.
[1]Stock N,Biswas S.Chem.Rev.,2012,112:933-969
[2]White K A,Chengelis D A,Gogick K A,et al.J.Am.Chem. Soc.,2009,131:18069-18071
[3]Alezi D,Belmabkhout Y,Suyetin Mil,et al.J.Am.Chem. Soc.,2015,137:13308-13318
[4]Brozek C K,Dinc M.J.Am.Chem.Soc.,2013,135:12886-12891
[5]Wang F,Liu Z S,Yang H,et al.Angew.Chem.Int.Ed., 2011,50:450-453
[6]Saha S,Das G,Thote J,et al.J.Am.Chem.Soc.,2014,136: 14845-14851
[7]Kim D,Whang D R,Park S Y.J.Am.Chem.Soc.,2016, 138:8698-8701
[8]Zheng B,Bai J,Duan J,et al.J.Am.Chem.Soc.,2011,133: 748-751
[9]WANG Hui(王慧),GAN Guo-Qing(甘国庆),QU Yang (瞿阳),et al.Chinese J.Inorg.Chem.(无机化学学报),2012, 28:1217-1221
[10]Cheng J J,Chang Y T,Wu C J,et al.CrystEngComm, 2012,14:537-543
[11]Shi X,Wang X,Li L,et al.Cryst.Growth Des.,2010,10: 2490-2500
[12]Xu Z,Wang Q,Li H,et al.Chem.Commun.,2012,48:5736-5378
[13]Xu Z,Meng W,Li H,et al.Inorg.Chem.,2014,53:3260-3262
[14]Xu Z,Li H,Li A,et al.Inorg.Chem.Commun.,2013,36: 126-129
[15]Pan J,Jiang F L,Wu M Y,et al.Cryst.Growth Des., 2014,14:5011-5018
[16]XU Zhou-Qing(徐周庆),WANG Xiao-Ning(王晓宁),LI Hui-Jun(李慧军),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32:1223-1230
[17]Sheldrick G M.SHELXS-97,Programs for X-ray Crystal Structure Solution,University of Göttingen,Germany,1997.
[18]SADABS,Bruker AXS Inc.,Madison,WI,2004.
[19]Meng W,Xu Z Q,Ding J,et al.Cryst.Growth Des.,2014, 14:730-738
[20]Silva C G,Corma A,García H.J.Mater.Chem.,2010,20: 3141-3156
基于不对称三氮唑衍生物配体的三个配合物的制备、晶体结构及光催化性能
徐周庆1何亚玲1李慧军*,1张培玲2王元*,1王奇1钟润斌1贾磊*,1
(1河南理工大学化学化工学院,焦作454000)
(2河南理工大学物理与电子信息学院,焦作454000)
利用水热法合成了基于不对称三氮唑衍生物配体Hptp(Hptp=2-(5-(pyridin-3-yl)-1H-1,2,4-triazol-3-yl)pyrazine)的3个配合物[Cd2(ptp)2(SO4)(H2O)2]n(1)、[Zn(ptp)2(H2O)2](2)和[Cd(ptp)2(H2O)2](3),它们的结构通过元素分析,红外,粉末X射线衍射和X射线单晶衍射表征。配合物1中,配体ptp-和Cu2+形成波浪状的一维结构,这些一维链通过SO42-链接形成三维结构。配合物2和3是同构化合物,单核单元在分子间氢键的作用下形成超分子二维平面。光催化降解实验表明,在双氧水存在时配合物1~3在60 min内使亚甲基蓝的降解率分别达到79%、81%和88%。
三氮唑衍生物;配合物;光催化;染料降解
O614.24+2;O614.24+1
A
1001-4861(2017)05-0897-08
2016-12-08。收修改稿日期:2017-03-13。
10.11862/CJIC.2017.097
国家自然科学基金(No.U1604124,21404033,21401046)、河南省科技攻关项目(No.152102210314)、河南省高等学校重点科研项目(No. 16A150010)和河南理工大学博士基金(No.72103/001/103)资助。
*通信联系人。E-mail:lihuijunxgy@hpu.edu.cn,wangyuan08@hpu.edu.cn,jlxj@hpu.edu.cn
- 无机化学学报的其它文章
- Two Tetrapodal Schiff Bases Acting as Colorimetric Sensors for Iron in Environmental Water Samples
- Syntheses,Characterization and Fluorescence Properties Analysis of Two Lanthanide Coordination Polymers Based on Axial Chirality Ligand 2,2′-Dinitro-4,4′-biphenyldicarboxylic Acid
- 磁性UiO-66复合材料的合成及其对水体中硝基酚有机分子的吸附性能
- cis-[(trans-1,2-双(氨甲基)环丁烷) (3-羟基-1,1-环丁烷二羧酸根)合铂(Ⅱ)]的合成和抗癌活性
- 两个具有Sn4O4梯状结构二丁基锡羧酸酯的微波溶剂热合成、结构和体外抗癌活性
- 混合配体构筑的配位聚合物的合成、结构与荧光性质

