Two Tetrapodal Schiff Bases Acting as Colorimetric Sensors for Iron in Environmental Water Samples
WANG RuoJIANG Guang-Qi*,1,
(1State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering,Key Laboratory of Green Pesticide and Agricultural Bioengineering,Ministry of Education,Guizhou University,Guiyang 550025,China)
(2College of Chemistry and Chemical Engineering,Guizhou University,Guiyang 550025,China)
Two Tetrapodal Schiff Bases Acting as Colorimetric Sensors for Iron in Environmental Water Samples
WANG Ruo2JIANG Guang-Qi*,1,2
(1State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering,Key Laboratory of Green Pesticide and Agricultural Bioengineering,Ministry of Education,Guizhou University,Guiyang 550025,China)
(2College of Chemistry and Chemical Engineering,Guizhou University,Guiyang 550025,China)
Two colorimetric sensors,derived from pentaerythrityltetramine and salicylaldehyde(1)or o-vanillin (2),had been synthesized.Both sensors showed selective colorimetric sensing ability for Feand Feby changing color from light yellow to either orange or purple when immersed in water samples.The sensors coordinated to Fewith 1∶1 stoichiometry,as has been confirmed from the results of LC-MS spectrometry and UV-Vis spectrum titration.The detection limits of sensors 1(0.19 μmol·L-1)and 2(0.21 μmol·L-1)are lower than the WHO guideline(5 μmol·L-1)in drinking water.
Schiff base;colorimetric sensor;iron
0 Introduction
According to recent research,the design and production of chemosensors for the detection of metal ions in aqueous solutions is an important area to develop,particularly with regards to sensors for the detection of heavy and transition metal cations[1-7]. Along with other transition metal ions,iron is an important element in living systems,as it plays crucial roles in the transport and storage of oxygen[8-9]. Iron is important because of its redox capacity in living systems,which can have a significant effect onhomeostasis[10-11].Iron can also promote development, increase resistance to disease,regulate tissue respiration,and prevent fatigue[12-13].Thus,to design chemosensors for the detection of Feand Fein an aqueous medium is of great significance.
Many analytical techniques have been used to determine the content of metal ions,such as plasma atomic emission spectrometry[14],atomic absorption spectroscopy[15],and fluorescence[16-17],electro-chemical[18], gravimetric[19-20],and chromatographic[21]methods.Other analytical techniques are described in[22-23].Most of thesemethodsrequiretheuseofsophisticated equipment,multi-step sample preparation,and trained operators,allofwhichleadtohighexpenses. Colorimetric sensors present the advantage of having a low cost and of being easy to use.No expensive equipment is required and the analyte is detectable by the naked eye.
Schiff bases are good ligands for metal ions,with whichtheycanformcoordinatebonds.This characteristic has resulted in their extensive use in the production of sensors.The reported chemosensors for Feand Fein aqueous solution are rare[24-35].In the present work,two tetrapodal Schiff bases with N and O donor atoms on the four pendant branches have beeninvestigatedtodeterminetheirabilityin detecting the presence of common metal cations.Both 1 and 2 showed selective colorimetric sensing ability for Feand Feby changing color from light yellow to either orange or purple when immersed in the water samples.The detection limits of sensor 1(0.19 μmol· L-1)and 2(0.21 μmol·L-1)are lower than the WHO guideline(5 μmol·L-1)for drinking water.The determination of the presence of Fein a variety of environmental water samples was investigated.
1 Experimental
1.1 Materials and instrumentation
Commerciallyavailablemetalsaltswere purchased from Aldrich and Alfa Aesar Chemical Co., Ltd.The solutions of metal ions were prepared from chloride or nitrate salts of Fe2+,Fe3+,Co2+,Ni2+,Cu2+, Zn2+,Mn2+,Cr3+,Cd2+,Pb2+,Hg2+,K+,Ca2+and Mg2+. Crystal structure was determined on a Bruker Apex 2 diffractometer.1H NMR and13C NMR(solvent CDCl3or DMSO-d6)spectral analyses were performed on a JEOL-ECX 500 NMR spectrometer at room temperature using TMS as an internal standard.LC-MS was determined on an Agilent LC/MSD Trap based on infusion methods.UV-Vis absorption spectra were recorded on a UV-1800 spectrophotometer(Beijing General Instrument Co.,China)in a 1 cm quartz cell.
1.2 Preparation of Schiff base
TheSchiffbases1and2wereprepared according to our previously described procedure[36-37].
1.3 Visual sensing of metal ions and UV-Vis study
Either sensor 1 or 2(0.005 mmol)was dissolved in DMSO(Dimethyl sulfoxide;50 mL).Fe2+,Fe3+,Co2+, Ni2+,Cu2+,Zn2+,Mn2+,Cr3+,Cd2+,Pb2+,Hg2+,K+,Ca2+and Mg2+(0.05 mmol)were dissolved in double-distilled water(50 mL),containing 1 mL of solution of either sensor 1 or 2;0.5 mL of each metal salt solution were transferred to a 10 mL volumetric flask and diluted to the final volume of 10 mL with DMSO and doubledistilled water to make the final solution(1∶5,nsensor/ nmetalcations and 1∶9,VDMSO/VH2O).Significant color changes(from light yellow to orange for 1,to purple for 2)could be observed by the naked eye.After mixing for a few minutes,UV-Vis spectra were takenat room temperature.
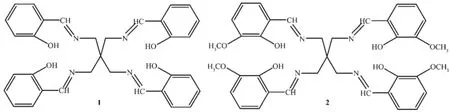
Fig.1Structures of sensors 1 and 2
1.4 Molar ratio method measurement of sensor 1 or 2 with Fe3+
Either sensor 1 or 2(0.05 mmol)was dissolved in DMSO(50 mL),Fe(NO3)3·9H2O(20.2 mg,0.05 mmol) was dissolved in double-distilled water(50 mL);0.1~2.0 mL of Fe(NO3)3solution were transferred to a 10 mL volumetric flask,1 mL of the solution of either sensor 1 or 2 was added subsequently,and diluted to 10 mL with DMSO and double-distilled water to make the final solution(1∶9,VDMSO/VH2O).After mixing for a few minutes,UV-Vis spectra were taken at room temperature.
1.5 Job plot measurement
Either sensor 1 or 2(0.05 mmol)was dissolved in DMSO(50 mL);0.1,0.2,0.3,0.4,0.5,0.6,0.7,0.8, and 0.9 mL of the solution of either sensor 1 or 2 were transferred to a 10 mL volumetric flask.Fe(NO3)3·9H2O (20.2 mg,0.05 mmol)was dissolved in double-distilled water(50 mL);0.9,0.8,0.7,0.6,0.5,0.4,0.3,0.2, and 0.1 mL of the Fe(NO3)3solution were added to each 10 mL volumetric flasks and diluted to 10 mL with DMSO and double-distilled water to make the final solution(1∶9,VDMSO/VH2O).After mixing for a few minutes,UV-Visspectraweretakenatroom temperature.
1.6 Competitive experiments
Sensor 1 or 2(0.05 mmol)was dissolved in DMSO (50 mL),and all the metal ions(0.05 mmol)were dissolved in double-distilled water(50 mL);1.0 mL of the solution of either sensor 1 or 2 solution was transferred to a 10 mL volumetric flask;1.0 mL of Fe(NO3)3solution and 1.0 mL of each other metal solution were added,then diluted to 10 mL with DMSO and doubledistilled water to make the final solution(1∶1∶1,nsensor∶∶nothermetal cations;1∶9,VDMSO/VH2O).After mixing for a few minutes,UV-Vis spectra were taken at room temperature.
1.7 Determination of Fe3+in environmental water samples
Environmental water samples were collected from a nearby river,water company,and spring water company.One milliliter of 1.0 mmol·L-1Fe(NO3)3solution was added to 8 mL of each environmental water sample using 10 mL volumetric flasks.Following this step,1 mL of the 1.0 mmol·L-1DMSO solution of either sensor 1 or 2 was added in each sample;9 mL of each environmental water sample was mixed with 1 mL of the 1.0 mmol·L-1DMSO solution of sensor 1 or 2 to carry out contrast experiments.The color changes of the artificial Fecontaining environmental water samples could be observed easily.
2 Results and discussion
2.1 Detection properties of sensor 1 and 2 toward Fe2+and Fe3+
We examined the detection abilities of sensors 1 and 2 in a series of metal ions such as Fe2+,Fe3+,Co2+, Ni2+,Cu2+,Zn2+,Mn2+,Cr3+,Cd2+,Pb2+,Hg2+,K+,Ca2+and Mg2+in a DMSO/H2O(1∶9,V/V)solution.Sensor 1 showed a significant color change,from light yellow to orange,in the presence of Fe2+or Fe3+,while other metalscausednocolorchangeunderidentical conditions(Fig.2a).Sensor 2 also showed a significant color change,from light yellow to purple,for Fe2+or Fe3+(Fig.2b).Fig.2c shows new absorption peaks at 482 nm and 488 nm in the presence of Fe2+and Fe3+, respectively,for sensor 1;Fig.2d shows new absorption peaks at 514 and 519 nm in the presence of Fe2+and Fe3+,respectively,for sensor 2.These results are consistent with the changes of color in the Fe2+and Fe3+solution.
The binding properties of sensors 1 and 2 with Fe3+were studied by UV-Vis titration.Upon the gradual addition of Fe3+(0.0~1.0 equiv)to a solution of either sensor 1 or 2,new absorbance peaks appeared.For sensor 1,the new peak formed at 488 nm and kept increasing with a clear isosbestic point at 338 nm (Fig.3a);for sensor 2,the new peak appeared at 519 nm and kept increasing with a clear isosbestic point at 345 nm(Fig.3b).These changes in the UV-Vis spectra clearly indicated that new species formed during the titration of sensor 1 or 2 with Fe3+.The new peaks at 488 and 519 nm with molar extinction coefficients of 6.1×103and 4.4×103L·mol-1·cm-1,respectively,are higher than those relative to the Febased d-d transitions.Thus,these new peaks might be attributedtometal-to-ligandcharge-transfer(MLCT)[38-39], which are responsible for the color changes of the solution.
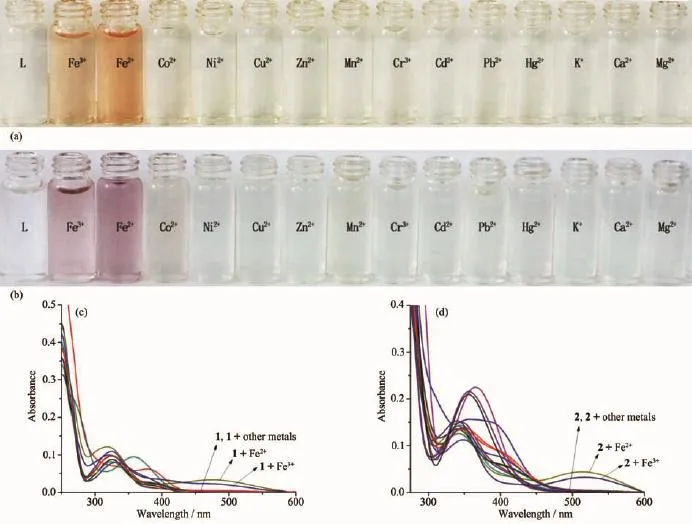
Fig.2(a)and(b)Color changes of sensors 1 and 2(50 μmol·L-1)upon addition of various metal ions(5 equiv)in DMSO/H2O (1∶9,V/V);(c)and(d)UV-Vis absorption spectra of sensors 1 and 2(10 μmol·L-1)in the presence of 5 equiv of different metal ions in DMSO/H2O(1∶9,V/V)
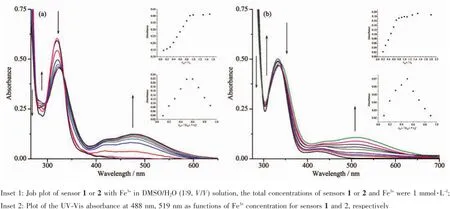
Fig.3UV-Vis absorption spectra of sensors 1(a)or 2(b)(0.1 mmol·L-1)with gradual addition of Fe3+(0~1 equiv)in DMSO/H2O (1∶9,V/V)solution
2.2 Evaluation of selectivity
Since selectivity is an important parameter in evaluating the behavior of an optical sensor,the interference effect of common coexistent metal ions was investigated on the detecting sensitivity of chemosensors towards Fe.The results reported in Fig.4a and 4b indicate that all the competing metal ions such as Ca2+,Cd2+,Co2+,Cr3+,Cu2+,Hg2+,K+,Mg2+,Mn2+, Ni2+,Pb2+,and Zn2+did not interfere noticeably with naked-eye detection of Fe3+.These results indicate that the tested sensors can act specifically for the detection of Fe3+.
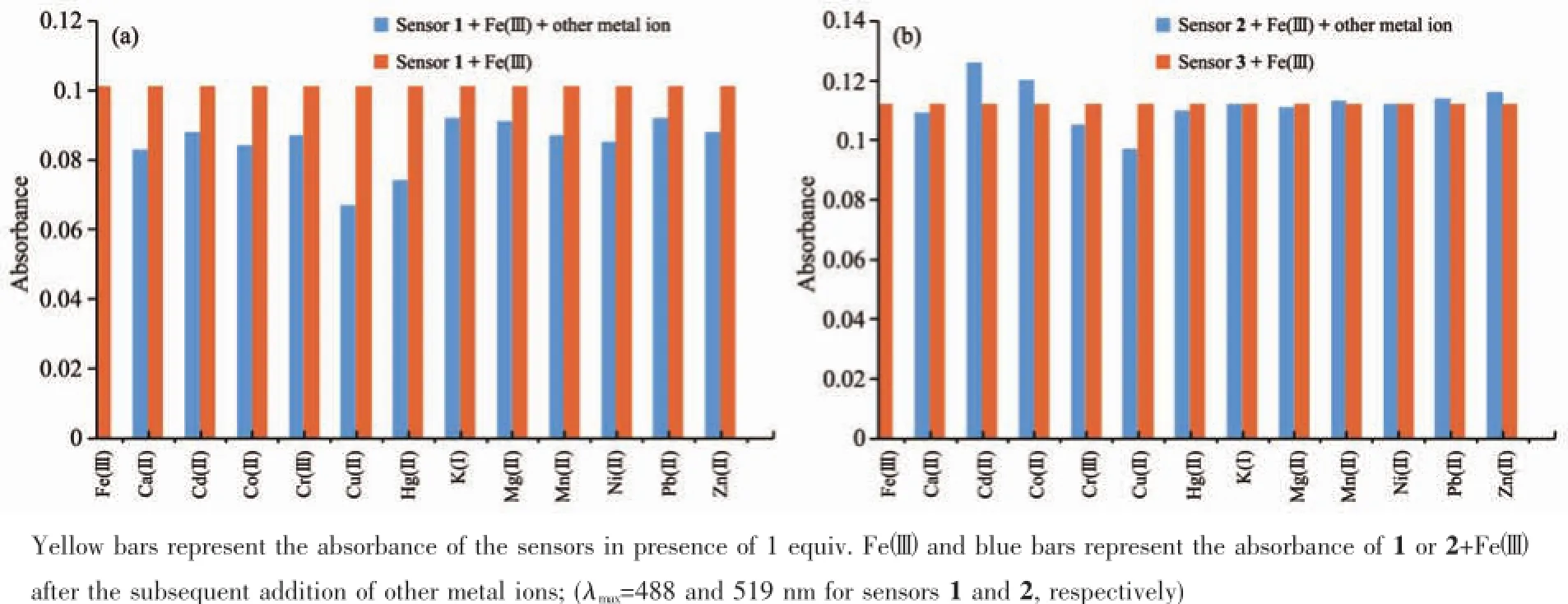
Fig.4Competitive selectivity of sensors 1(a)or 2(b)(0.1 mmol·L-1)towards various metal ions(0.1 mmol·L-1)
2.3 Stoichiometry and binding mode studies
The stoichiometry of metal complexes formed between the sensors and Fe3+ions was determined by Job′s titration method and the molar ratio method[40]. As shown in Fig.3a and Fig.3b,the maximum values of the absorbance curves were obtained at a 0.5 molar ratio,indicating 1∶1 stoichiometry of the resulting Fecomplexes.The molar ratio method also showed the same results for these complexes.The stoichiometries of these complexes were further confirmed by LC-MS spectrometry analysis.The positive-ion mass spectrum of the sensors upon addition of 1 equiv of Fe3+showed the formation of the sensor 1-3H++Fe3+complex(m/z: 722.3;Calcd.,722.2)and the sensor 2-3H++Fe3+complex(m/z:602.2;Calcd.,602.15)(Fig.5a and 5b, respectively).The crystal structure of sensor 1-3H++ Fe3+complex had also been obtained in our earlier work[36],which had clearly revealed the 1∶1 stoichiometry of the Fecomplex(Fig.5c).In this crystal structure, the Feion coordinated with imine nitrogen atoms (N(1),N(2),N(3))and phenolic oxygen atoms(O(1), O(2),O(3))from three pendant branches of sensor 1, locatedatthecenterofoctahedralcoordination sphere;meanwhile,one of the salicylaldimine pendant branches remained uncoordinated,and the phenolic OH and the imine nitrogen were intramolecularly hydrogen-bonded.Based on the UV-Vis titration,Job plot,and LC-MS analysis,as well as the crystal structure analysis,we confirmed the structures of the 1-Fe3+and 2-Fe3+complexes with 1∶1 stoichiometry.In addition,the binding constants(Ka)of sensors 1 and 2 with Fe3+were calculated to be equal to 1.8×104and 8.5×104,respectively,on the basis of Benesi-Hildebrand analysis.These values fall well within the range of 103~105,as previously reported for other Fesensors[2,41]. The detection limits of sensors 1 and 2 for Fe3+ions were determined as 0.19 and 0.21 μmol·L-1,respectively,based on the IUPAC definition(CDL=3Sb/m),well below the WHO guideline(5 μmol·L-1)for Fe3+in drinking water.It is worth mentioning that the workingcurves for measurement of Fe3+showed good linear relationships within the concentration range of 3~33 μmol·L-1for both sensors(Fig.6a and 6b).These results suggest that the tested sensors 1 and 2 can be adequately employed as probes for the detection of micromolar concentrations of Fe3+in real water samples.
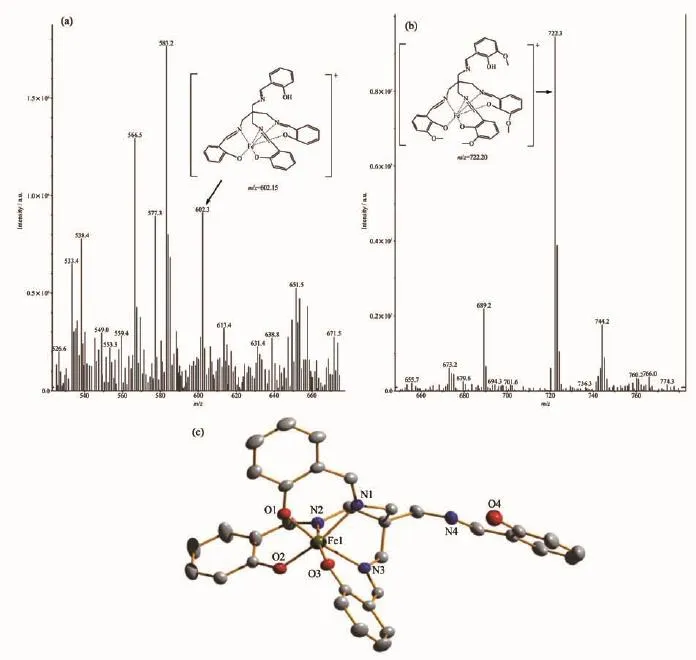
Fig.5(a)Positive-ion electrospray ionization mass spectrum of sensor 1(50 μmol·L-1)upon addition of 1 equiv.of Fe(NO3)3in acetonitrile solution;(b)Positive-ion electrospray ionization mass spectrum of sensor 2 (50 μmol·L-1)upon addition of 1 equiv of Fe(NO3)3in acetonitrile solution;(c)Crystal structure of the sensor 1-3H++Fe3+complex
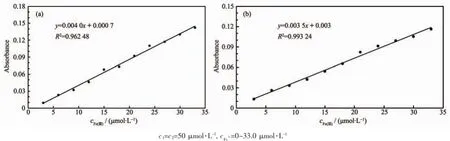
Fig.6Absorbance at 488 and 519 nm for sensor 1(a)and sensor 2(b)as a function of Fe3+concentration
2.4 Effect of pH value
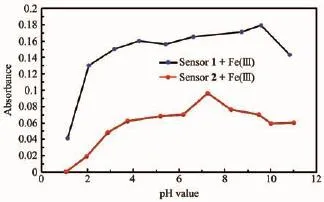
Fig.7pH value sensitivity of Fe3+detection of sensors 1 and 2
The sensitivity of the colorimetric sensor complexes(1-Fe3+or 2-Fe3+)at different pH values was studied in a DMSO/H2O(1/9,V/V)solution.Experiments were conducted by adjusting pH using NaOH and HCl.As depicted in Fig.7,both sensors were found to be quite effective in the neutral pH range.In an acidic medium,the absorbance of 1-Fe3+or 2-Fe3+complexes decreased due to the decomposition of the Fecomplexes;while in a basic medium,the absorbance decreased because of the formation of the Fe(OH)3colloid.These results suggest that these colorimetric sensors are suitable for application under the biologically relevant pH range of 6.0~7.6.
2.5 Application studies
To determine the applicability of the proposed sensors in a real-case scenario,we examined their colorimetricbehaviorin the detection of Fe3+in different environmental water samples,under the same conditions.As shown in Fig.8a and 8b,the changes in color of the environmental water samples are clearly observable when even trace levels of Fewere present. Furthermore,recovery experiments of eighteen samples were conducted at three different levels of the Feconcentration for river water,tap water and spring water,respectively,usingthe standardcurve method[42-44]. As shown in Table 1,the results showed satisfactory recovery and R.S.D.values for these water samples, this indicates that the sensors 1 or 2 could potentially be used for the determination of the Fe3+ion in environmental water samples.Furthermore,it is well known that Fe2+ion can easily be oxidated to Fe3+ion when exposed to air.It is possible for the determination of the Fe2+ion after its oxidation to the Fe3+ion using the same procedure as described for the Fe3+ion.
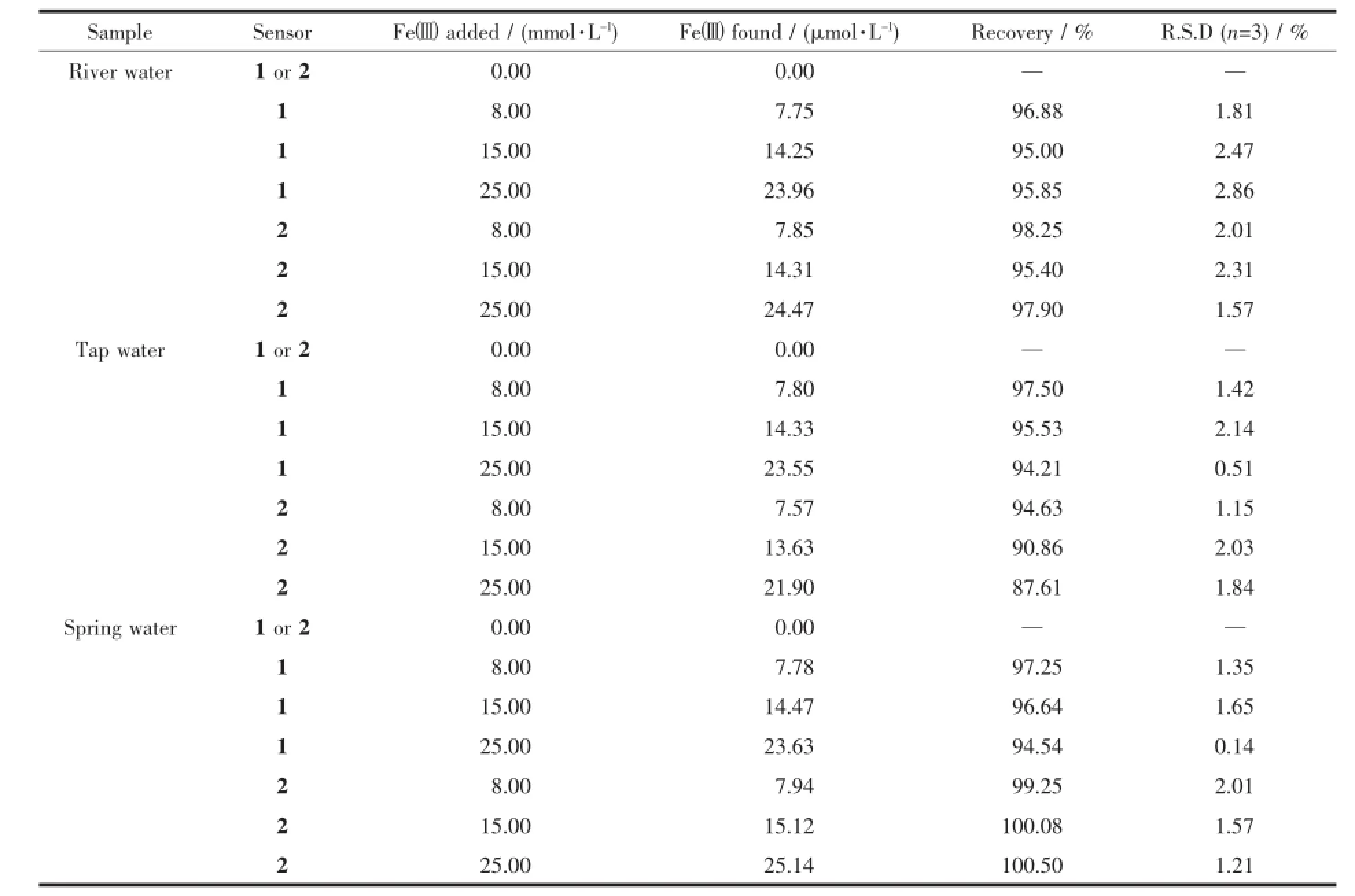
Table 1Determinatin of Fe3+in water samples*
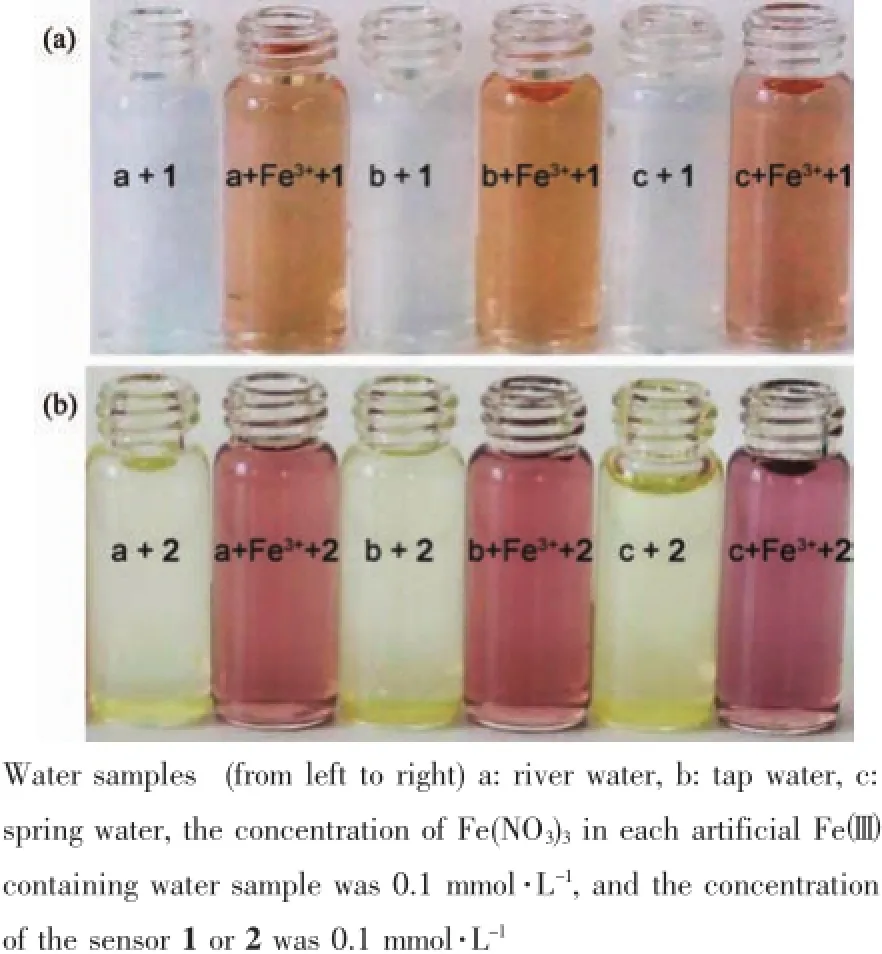
Fig.8Determination of Fe3+in environmental water samples using sensor 1(a)or 2(b)as colorimetric sensors
3 Conclusions
Insummary,twocolorimetricsensorswere developed for the detection of Fe2+and Fe3+in aqueous media.They exhibited excellent selectivity towards these two ions over other metal cations commonly found in water.The 1∶1 stoichiometries of the Fecomplexes have been confirmed by X-ray crystallography,LC-MS spectrometry analysis,and UV-Vis spectrum titration.The detection limits of sensor 1 (0.19 μmol·L-1)and 2(0.21 μmol·L-1)for Fe3+are far below the WHO guidelines(5 μmol·L-1).Both sensors 1 and 2 were found to be quite effective in the neutral pH value range and showed good linear relationships within the 3~33 μmol·L-1concentration range for the measu-rement of Fe3+.The practical applicability studies showed satisfactory recovery and R.S.D.values for the detection of Fe3+ions in environmental water samples.
Acknowledgements:Wearethankfulforfinancial support from the Foundation of Guizhou Province,China(Grant No.[2014]7619);the Teaching Reform and Research Project of the Guizhou University,China(Grant No.JG 2013014);the science and technology innovation foundation of the Guizhou University(Grant No.2016009);the opening foundation of the Key Laboratory of Green Pesticide and Agricultural Bioengineering,Ministry of Education,Guizhou University(Grant No. 2016GDGP0102).
[1]Callan J F,Silva A P,Magri D C.Tetrahedron,2005,61: 8551-8588
[2]Sen S,Sarkar S,Chattopadhyay B,et al.Analyst,2012,137: 3335-3342
[3]Zhu J,Yuan H,Chan W,et al.Org.Biomol.Chem.,2010,8: 3957-3964
[4]Valeur B,Leray I.Chem.Rev.,2000,205:3-40
[5]Shellaiah M,Wu Y H,Lin H C.Analyst,2013,138:2931-2942
[6]Lee D Y,Singh N,Jang D O,et al.Tetrahedron Lett.,2010, 51:1103-1106
[7]H Kim,Na Y J,Song E J,et al.RSC Adv.,2014,4:22463-22469
[8]Berthon G.Handbook of Metal-Ligand Interactions in BiologicalFluids:BioinorganicMedicine:Vol.1.NewYork:Dekker, 1995.
[9]Bertini I,Gray H B,Lippard S J,et al.Bioinorganic Chemistry.Mill Valley:University Science Book,1994.
[10]Yang Z,Yan C,Chen Y,et al.Dalton Trans.,2011,40:2173 -2176
[11]Kim K B,Kim H,Song E J,et al.Dalton Trans.,2013,42: 16569-16577
[12]Crichton R R,Wilmet S,Legssyer R,et al.Inorg.Biochem., 2002,91:9-18
[13]Philpott C C.J.Biol.Chem.,2012,287:13518-13523
[14]Nomngongo P N,Ngila J C.Spectrochim.Acta B,2014,98: 54-59
[15]Caldas L,Brum D M,Paula C.Talanta,2013,110:21-27
[16]Lai T T,Zheng E H,Chen L X.Nanoscale,2013,5:8015-8021
[17]Kaya E N,Yuksel F,Ozpinar G A,et al.Sens.Actuators B, 2014,194:377-388
[18]Oh J W,Kim T H,Yoo S W.Sens.Actuators B,2013,177: 813-817
[19]Potts L W.Quantitative Analysis.New York:Theory and Practice,1987:656
[20]Arthur I V.Textbook of Macro and Semimicro Qualitative Inorganic Analysis.New York:Longman,1979:605
[21]Harvey D.Modern Analytical Chemistry.New York:Wiley, 2000:816
[22]Zhang Z Y,Lu S Z,Sha C M,et al.Sens.Actuators B,2015, 208:258-266
[23]Kozak J,Jodlowska N,Kozak M,et al.Anal.Chim.Acta, 2011,702:213-217
[24]You G R,Park G J,Lee S A.Sens.Actuators B,2015,215: 188-195
[25]Li P,Zhao Y,Yao L,et al.Sens.Actuators B,2014,191: 332-336
[26]Li S H,Li Y C,Cao J.et al.Anal.Chem.,2014,86:10201-10207
[27]Nandre J,Patil S,Patil V,et al.Biosens.Bioelectron.,2014, 61:612-617
[28]Goel A,Umar S,Nag P,et al.Chem.Commun.,2015,51: 5001-5004
[29]Chen Y J,Yang S C,Tsai C C,et al.Chem.Asian J.,2015, 10:1025-1034
[30]Chereddy N R,Nagaraju P,Raju M,et al.Biosens.Bioelectron.,2015,68:749-756
[31]Ju J,Chen W.Biosens.Bioelectron.,2014,58:219-225
[32]Yang L,Yang W,Xu D M,et al.Dyes Pigm.,2013,97:168-174
[33]Wang J H,Zhang D,Liu Y Q,et al.Sens.Actuators B, 2014,191:344-350
[34]Wang C C,Zhang D,Huang X Y,et al.Talanta,2014,128: 69-74
[35]Wang M,Wang J G,Xue W J.Dyes Pigm.,2013,97:475 -480
[36]Jiang G Q,Cai J,Zhang Y Q,et al.J.Struct.Chem.,2012,31: 385-388
[37]Jiang G Q,Cai J,Zhang Y Q,et al.Acta Crystallogr.Sect. E,2008,E64:o1455
[38]Meier M,Schubert U S.Chem.Commun.,2005,36:4610-4612
[39]Choi Y W,Park G J,Na Y J,et al.Sens.Actuators B,2014, 194:343-352
[40]Job P.Ann.Chim.,1928,9:113-203
[41]Kim H,Kim K B,Song E J,et al.Chem.Comm.,2013,36: 72-76
[42]Wang Z H,Qin Y X,Wang C,et al.Appl.Surf.Sci.,2012, 258:2017-2021
[43]Dong L,Wu C,Zeng X.Sens.Actuators B,2010,145:433-437
[44]Zhou Y M,Zhang J L,Zhou H.Spectrochim.Acta Part A, 2013,106:68-72
两种四足席夫碱对环境水样中Fe离子的比色识别
王若2江光奇*,1,2
(1贵州大学精细化工研究开发中心绿色农药和农业生物工程教育部重点实验室,贵阳550025)
(2贵州大学化学与化工学院,贵阳50025)
使用季戊四胺与水杨醛、邻香兰素缩合反应得到了两种席夫碱化合物1和2。化合物1和2均可用于不同水样Fe和Fe离子的比色识别。通过光谱滴定及液质谱联用等方法研究了化合物1和2与Fe的识别作用,化合物1和2与Fe离子的配位比为1∶1,对Fe铁离子的检出限分别为0.19和0.21 μmol·L-1,低于世界卫生组织饮用水检出标准(5 μmol·L-1),具有一定的实用价值。
席夫碱:比色传感器:铁离子
O614.81+1
A
1001-4861(2017)05-0881-09
2016-11-02。收修改稿日期:2017-02-04。
10.11862/CJIC.2017.079
贵州省联合基金项目(No.黔科合LH字[2014]7619)、贵州大学教改项目(No.JG2013014)、贵州大学研究生创新基金(No.2016009)和贵州大学绿色农药与农业生物工程教育部重点实验室开放基金(No.2016GDGP0102)资助。
*通信联系人。E-mail:gqjiang@163.com
- 无机化学学报的其它文章
- 火焰辅助热解方法制备Ag2O/TiO2及其光催化制氢性能
- Tm3+/Er3+/Ho3+共掺SiO2-Bi2O3-AlF3-BaF2玻璃发光性能
- 一种三足四齿Schiff碱的Cu(Ⅱ)、Co(Ⅲ)配合物的合成、晶体结构与表征
- Syntheses, Crystal Structures and Insulin-like Activity of Maltolato- and Ethylmaltolato-Coordinated Oxovanadium(Ⅴ) Complexes Derived from 4-Fluoro-N′-(3-ethoxy-2-hydroxybenzylidene)benzohydrazide
- Electrochemically Self-Assembled Fe/Cu Nanocomposite with Improved High-Rate and Low-Temperature Performances for Nickel-Iron Alkaline Battery
- 碳化硅衍生碳的制备及其超级电容性能

