青杄MYB转录因子基因PwMYB20的克隆及表达分析*
游韩莉 袁义杭 李长江 张凌云
(北京林业大学 省部共建森林培育与保护教育部重点实验室 北京 100083)
青杄MYB转录因子基因PwMYB20的克隆及表达分析*
游韩莉 袁义杭 李长江 张凌云
(北京林业大学 省部共建森林培育与保护教育部重点实验室 北京 100083)
【目的】 MYB转录因子家族是植物中最大的一类转录因子,在植物生长发育及抗逆调控网络中发挥重要作用。对青杄中MYB同源基因PwMYB20的克隆与分析,有利于进一步探究PwMYB20在植物生长发育及逆境响应中的功能,挖掘与利用青杄中的优质基因。【方法】 采用RACE-PCR技术,从青杄cDNA文库中克隆得到PwMYB20基因,并通过PCR技术克隆验证。利用ProtParam、ProtScale、FoldIndex等生物信息学软件对PwMYB20理化性质进行分析预测。通过BLAST在线工具得到植物同源蛋白,并对其进行比对分析和进化树分析。采用实时荧光定量PCR技术分析PwMYB20基因在不同组织中的表达性,以及干旱、低温、盐、ABA等非生物逆境胁迫处理后的表达变化。通过亚细胞定位及转录激活活性验证试验,揭示其生物学特性。【结果】 通过RACE-PCR克隆得到PwMYB20 cDNA全长966 bp,含675 bp的完整开放阅读框,编码225个氨基酸。ProtParam工具计算蛋白分子式为C1104H1740N340O330S8,分子质量为25.3 kDa,等电点为9.11; Protscale工具疏水性分析发现,PwMYB20的疏水位点与亲水位点均匀分布,推测该蛋白为亲水蛋白; SignalP工具预测发现该蛋白没有信号肽结构域; 利用FoldIndex工具对蛋白质固有无序化进行分析,结果表明该蛋白固有无序化序列较多,推测在生理环境下蛋白的动态活性较大; TMHMM工具预测发现该蛋白没有跨膜结构域。通过对比分析发现PwMYB20属于MYB家族基因,编码1个R2R3-MYB蛋白。进化树分析结果显示,青杄PwMYB20与白云杉PgMYB20聚为一簇。实时荧光定量PCR结果表明,PwMYB20在种子中的表达量最高,其次是在针叶中,在花粉中的表达相对较少。PwMYB20对干旱、4 ℃和ABA处理均有响应,而对NaCl处理响应相对较弱。在干旱处理下,PwMYB20表达量先上升后下降;4 ℃低温处理3 h和12 h时PwMYB20的表达量上升,在4 ℃处理6 h时存在波动,呈现上升—下降—上升的趋势;PwMYB20的表达受ABA处理持续诱导。亚细胞定位分析表明,PwMYB20是一个主要定位于细胞核中的蛋白质。转录激活活性分析结果显示,PwMYB20的C端存在转录激活活性,而PwMYB20全长及其N端没有转录激活活性。【结论】 青杄PwMYB20,作为一个转录因子发挥作用,其转录激活活性位于C端; 受干旱、低温和ABA诱导,普遍参与了植物应对逆境胁迫的响应过程。
青杄; MYB转录因子; 基因克隆; 胁迫响应; 基因表达
转录因子是指能特异性结合靶基因启动子上顺式作用元件的蛋白质,对转录起着激活或抑制作用。在植物生长发育及抗逆调控网络中,转录因子发挥了十分重要的作用(Broun, 2004; Wangetal., 2016)。MYB家族转录因子是一类含有长为50~53个氨基酸残基且高度保守的MYB结构域的转录因子,是植物转录因子中最大的转录因子家族。Paz-Ares等(1987)在玉米(Zeamays)中鉴定出第1个植物MYB基因,并命名为C1。之后,越来越多的MYB相关基因被分离鉴定。
MYB转录因子在结构上具有由1~4个MYB重复单元(R)组成的MYB结构域。根据R的个数,可以将其分为4个亚类,即只含1个R的MYB-related类型、包含2个R的R2R3-MYB类型、包含3个R的3R-MYB类型以及包含4个R的4R-MYB类型(Strackeetal., 2001; Dubosetal., 2010)。在植物中,R2R3-MYB类型MYB蛋白数量较多(Chenetal., 2006),且参与了植物的初生和次生代谢、植物细胞形态和模式建成、植物生长发育等多个生命过程的调节(Dubosetal., 2010; Ambawatetal., 2013)。拟南芥(Arabidopsisthaliana)中MYB11、MYB12和MYB111通过调控FLAVONOLSYNTHASE1等下游靶基因,调节类黄酮的生物合成(Strackeetal., 2007)。在许多物种中均鉴定到参与细胞壁形成的MYB转录因子,例如小麦(Triticumaestivum)的TaMYB4和玉米的ZmMYB31(Fornaleetal., 2010; Maetal., 2011)。此外,MYB对种子胚的发育、根系的发育等均有调控作用(Tominagaetal., 2008; Yangetal., 2009)。
此外,研究发现MYB家族基因也参与了植物逆境响应过程。干旱胁迫能显著诱导拟南芥AtMYB2的表达,在植物受到干旱胁迫时,AtMYB2可以激活一些受ABA诱导表达基因的转录,从而提高对干旱胁迫的耐受程度(Uraoetal., 1993; Abeetal., 2003)。AtMYB44是一个R2R3类型的MYB转录因子,参与调节植物气孔闭合和植物对非生物胁迫的响应(Jungetal., 2008)。进一步研究发现,AtMYB44通过直接与WRKY70结合,抑制茉莉酸介导的防卫反应,激活水杨酸介导的防卫反应(Shimetal., 2013)。此外,水稻(Oryzasativa)中OsMYB2和小麦中的TaMYB4在植物响应生物胁迫与非生物胁迫中均发挥了重要作用(Yangetal., 2012; Al-Attalaetal., 2014)。
青杄(Piceawilsonii)是松科(Pinaceae)云杉属(Picea)的一种常绿高大乔木,高可达50 m,是我国特有的针叶树种。其适应能力强,喜阴且极耐寒,多分布于凉爽湿润地区。目前,青杄已被多个地区列为水源涵养林及用材林的主要造林和更新树种。早期对青杄的研究多集中于青杄的群落生态学(张大勇等, 1989),随后有研究者对青杄愈伤组织的诱导(杨映根等, 1994)及育苗栽培(许家春等, 2004)也进行了相关研究。近年来,随着分子生物学和细胞生物学的快速发展,对于青杄基因的功能研究也有了一定进展。本文通过RACE-PCR的方法,从青杄cDNA文库中克隆得到1个R2R3-MYB转录因子全长cDNA序列; 采用荧光定量PCR分析其在青杄各组织以及各种非生物胁迫下的表达情况,并进一步利用亚细胞定位及转录激活活性验证试验揭示其生物学特性,为进一步探究其在植物生长发育及逆境响应中的功能打下基础,同时有利于挖掘与利用木本植物中的优质基因。
1 材料和方法
1.1 试验材料及处理方法
青杄花粉及种子均采集于北京植物园。3年生青杄幼苗的根、茎、针叶与花粉用于组织特异表达试验,将青杄种子播种于体积1∶1蛭石和草炭土的培养基质中,置于温度21 ℃、相对湿度60%~70%、日照时间16 h的条件下培养,每周定时浇1次水。8周的青杄幼苗用于逆境响应试验。
逆境响应试验的处理方法参照张通等(2014)和李长江等(2014)略有改动: 将8周的青杄幼苗裸根置于室温(25 ℃),于吸水纸上放置0,3,6,12 h; 用100 mmol·L-1NaCl处理青杄幼苗0,3,6,12 h; 将青杄幼苗于清水中4 ℃处理0,3,6,12 h。为了验证PwMYB20对逆境胁迫的响应是否通过ABA途径,进行了外施ABA试验,即用100 μmol·L-1ABA分别处理青杄幼苗0,3,6,12 h。
1.2 青杄PwMYB20全长cDNA的获得
青杄cDNA文库通过Gateway方法构建,由Invitrogen(上海)公司完成(张盾等, 2012)。在前期构建的多年生青杄均一化cDNA文库的基础上,利用RACE-PCR的方法得到PwMYB20末端序列,再经过序列拼接得到cDNA全长。RACE-PCR所用引物为MYB20-RACE-F、MYB20-RACE-R(表1)。设计引物MYB20-F、MYB20-R从青杄cDNA文库克隆得到PwMYB20的ORF,连接到pEASY-T1上,获得PwMYB20单克隆。
1.3 PwMYB20生物信息学分析
PwMYB20生物信息学分析方法参照李长江等(2014)。利用DNAMAN软件进行PwMYB20的cDNA序列编码区预测及蛋白翻译。运用ProtParam工具(http: //biopython.org/wiki/ProtParam)分析蛋白的理化特性和氨基酸组成。通过BLAST工具在NCBI(https: //www.ncbi.nlm.nih.gov/)上进行核酸序列与蛋白序列的同源性分析。利用ClustalX软件进行氨基酸多序列比对分析,并用MEGA5软件基于邻位相连法构建系统发育树。利用WoLF-PSORT(http: //www.genscript.com/tools/wolf-psort)进行蛋白亚细胞定位预测。通过Protscale(http: //web.expasy.org/protscale/)、SignalP4.1(http: //www.cbs.dtu.dk/services/SignalP/)、TMHMM(http: //www.cbs.dtu.dk/services/TMHMM/)、FoldIndex(http: //bip.weizmann.ac.il/fldbin/findex)对蛋白疏水性、信号肽区域、跨膜结构域以及蛋白无序化位点进行预测分析。
1.4PwMYB20组织特异性表达及逆境响应分析
利用艾德莱(北京)公司的植物RNA快速提取试剂盒提取各试验材料的RNA,并用天根(北京)公司反转录试剂盒合成第1条cDNA链,置于-20 ℃保存。根据基因序列的非保守区设计定量引物为qMYB20-F、qMYB20-R。选取青杄EF1-α基因作为内参基因(李长江等, 2014),引物为EF1-α-F、EF1-α-R。将合成的cDNA第1条链均一化浓度之后在StepOnePlus Real Time RT-PCR仪器上进行荧光定量PCR,引物序列详见表1。试验分别设置2次生物学重复,3次技术重复。利用2-△△Ct法分析数据,用SPASS与SigmaPlot分析作图。

表1 所用引物序列Tab.1 Primer sequences
1.5 PwMYB20瞬时表达载体构建与亚细胞定位

图1 pEZS-NL的载体Fig.1 Map of pEZS-NL35S表示启动子序列; Ala10表示10个丙氨酸序列; EGFP表示GFP序列; Ocs3′表示章鱼碱合成酶基因的终止子。35S shows promoter sequence; Ala10 shows 10 alanine sequences; EGFP shows GFP sequence; Ocs3′ shows terminator.
通过引物MYB20-F-KpnⅠ、MYB20-R-BamHⅠ(表1)扩增PwMYB20编码区序列,扩增产物经双酶切后定向连入pEZS-NL载体(图1)。构建好的表达载体转化大肠杆菌(Escherichiacoli)菌株DH5α。利用天根(北京)质粒大型大量提取试剂盒提取质粒,质粒浓度至少达到1 μg·μL-1。通过基因枪将质粒轰击入洋葱(Alliumcepa)表皮细胞(Yuetal., 2011)。将转化的洋葱表皮置于MS培养基上暗培养1天,用共聚焦显微镜(OLYMPUS,FV10i)观察拍照。为了确定PwMYB20表达部位,用空pEZS-NL载体所表达的绿色荧光以及DAPI荧光作为参照。
1.6 PwMYB20转录激活活性检测
设计引物,并在上游和下游引物分别加入EcoRⅠ和BamHⅠ酶切位点,将PwMYB20全长(1-225 aa)、N端(1-127 aa)和C端(133-225 aa)分别连入pGBKT7载体,引物详见表1。构建好的表达载体转化大肠杆菌菌株DH5α,并测序验证。利用天根(北京)公司质粒小提试剂盒提取重组质粒,存于-20 ℃备用。将经测序验证正确后的重组质粒转入酵母AH109(Saccharomycescerevisiae)菌株,操作按照上海唯地生物技术有限公司说明书进行。以转入空pGBKT7载体为阴性对照,转入pGBKT7-ANAC092为阳性对照(Heetal., 2005),均匀涂于SD-Trp单缺陷平板上。生长2~3天后用接种针挑取少量的酵母单克隆,混于500 mL无菌水中,吸取5 μL滴在SD-Trp和含有10 mmol·L-13-氨基-1, 2, 4-三氮唑(3-AT)的SD-Trp-His-Ade缺陷型酵母培养基上。培养3~4天后,若在SD-Trp-His-Ade缺陷型酵母培养基上长出菌落,则具有转录激活活性,反之则无。
2 结果与分析
2.1 基因克隆与序列分析
用RACE-PCR的方法得到末端序列,与EST序列拼接,得到PwMYB20的cDNA全长(图2)。利用DNAMAN软件分析发现,PwMYB20的cDNA全长为966 bp,在86 bp处出现起始密码子ATG,在741 bp处出现终止密码子TGA,编码区共675 bp,编码225个氨基酸,在949 bp处出现PolyA尾巴。

图2 PwMYB20的核酸序列与蛋白氨基酸序列Fig.2 Nucleotide sequence and deduced protein amino acids sequence of PwMYB20起始密码子(ATG)与终止密码子(TGA)用下划线标记,氨基酸序列用单字母表示,黑体字母表示PolyA尾巴。Potential translation initiation codon (ATG) and termination codon (TGA) are underlined, amino acid residues are indicated by single letter code and PolyA tail are indicated by bold letters.

图3 PwMYB20的理化特性分析Fig.3 Physicochemical properties of PwMYB20A: Protscale工具预测蛋白疏水性; B: PwMYB20的信号肽预测(C-score表示剪切位置分值,S-score表示信号肽分值,Y-score表示综合剪切位置分值); C: PwMYB20蛋白的固有无序化分析; D: PwMYB20的跨膜结构域分析,图中第1条横线(1.0~1.2)表示综合结果。A: Hydrophobic analysis of PwMYB20; B: Signal peptide analysis of PwMYB20(C-score means Cleavage site score, S-score means Signal peptide score, Y-score means Combined cleavage site score); C: Intrinsically disordered protein analysis of PwMYB20; D: Transmembrane analysis of PwMYB20,the first horizontal line(1.0-1.2)in the graph represents the comprehensive result.

图4 PwMYB20及同源蛋白的多序列比对Fig.4 Multiple sequence alignment of PwMYB20 and homological proteins线段示意不同结构域(R2,R3,TAD),虚线框示意青杄等物种中特有的结构域。相似性: 黑色=100%; 粉色≥75%; 蓝色≥50%。The lines indicate different domains (R2, R3, TAD), the dashed box indicates a specific domain in Picea wilsonii et al. Conserved percent: Black=100%; Pink≥75%; Blue≥50%.青杄Picea wilsonii:PwMYB20; 白云杉Picea glauca:PgMYB10(ABQ51226.1), PgMYB5 (ABQ51221.1), PgMYB17 (ACN12959.1), PgMYB13(ABQ51229.1); 火炬松Pinus taeda:PtMYB14(ABD60279.1); 拟南芥Arabidopsis thaliana:AtMYB6 (NP_192684.1), AtMYB7(NP_179263.1),AtMYB32(NP_195225.1),AtMYB4(NP_195574.1).
利用ProtParam工具计算蛋白分子式为C1104H1740N340O330S8,分子量为25.3 kDa,等电点为9.11。亮氨酸(Leu)含量最高(9.8%),其次为8.9%的丝氨酸(Ser)与8.0%的精氨酸(Arg)。预测在体外半衰期为30 h,不稳定指数为55.02,说明蛋白不稳定。Protscale工具疏水性分析发现,苯丙氨酸(Phe81)分值最大,为1.633,谷氨酰胺(Gln20)分值最小,为-2.647。疏水位点与亲水位点均匀分布,推测该蛋白为亲水蛋白(图3A)。SignalP工具预测发现该蛋白没有信号肽结构域(图3B)。利用FoldIndex工具对蛋白质固有无序化进行分析,结果表明该蛋白固有无序化序列较多,推测在生理环境下蛋白的动态活性较大(图3C)。TMHMM工具预测发现,整条肽链都位于膜外,因此推测该蛋白没有跨膜结构域(图3D)。此外,利用WOLF-PSORT对PwMYB20亚细胞定位进行预测,发现其可能定位在细胞核中。
2.2 多重序列比对及系统进化树分析
通过BLAST工具在NCBI上用翻译后的蛋白序列进行检索,结果表明其属于R2R3-MYB家族,且在N端发现SANT结构域。选取拟南芥、白云杉(Piceaglauca)等物种同源基因进行检索,发现PwMYB20与其他MYBs相似性较高。利用ClustalX工具进行多序列比对,发现PwMYB20与其他物种的MYBs在N端有较高的相似性,且为R2R3结构域,在C端则拥有比较特异的转录激活域TAD。与拟南芥相比,C端的结构域是青杄、白云杉等物种特有的(图4)。
利用MEGA5软件的邻位相连法进行系统发育树的构建,可以发现PwMYB20与PgMYB20聚为一簇,拟南芥的MYBs与木本植物的MYBs明显分为两大类(图5),进而推测2类的激活域TAD拥有不同的分子功能。

图5 PwMYB20的系统发育树分析Fig.5 Phylogenetic tree analysis of PwMYB20MEGA5用于构建系统发育树,计算方法为邻位相连法。每个分支上的数字表示1 000次重复搜索的置信度。MEGA5 is applied to construct the tree by Neighbor-joining method. Numbers on branches indicate bootstrap estimates for 1 000 replicate analysis.
2.3PwMYB20组织特异表达分析
利用RT-qPCR试验检测PwMYB20在青杄各组织中的表达模式,结果发现PwMYB20在青杄的各个组织中均有表达,在种子中的表达量最高,其次是在针叶中,在花粉中的表达相对较少(图6)。说明PwMYB20基因属于组成型表达,可能对青杄种子、针叶、花粉、茎和根的发育均有影响。
2.4 逆境胁迫对PwMYB20表达的影响
为了研究不同胁迫条件下PwMYB20的表达模式,取胁迫处理后的整株青杄幼苗,提取RNA后进行反转录,浓度均一化后进行荧光定量PCR试验。结果表明,PwMYB20对干旱、4 ℃和ABA处理均有响应,而NaCl处理对PwMYB20表达的影响相对较弱(图7)。在干旱处理下,PwMYB20表达量先上升后下降,在处理6 h后表达量最高,为未处理幼苗表达量的7倍。4 ℃低温处理3 h和12 h时PwMYB20的表达量显著高于未处理的幼苗,而4 ℃处理6 h时PwMYB20的表达量并没有明显上升,呈现上升—下降—上升的趋势。ABA处理显著提高了PwMYB20的表达量,其表达量持续上升,处理12 h后PwMYB20的表达量是未处理幼苗表达量的8倍。
2.5 PwMYB20的亚细胞定位分析
pEZS-NL载体是CaMV 35S驱动的植物瞬时表达载体,具有GFP表达序列(图1)。将PwMYB20构建在pEZS-NL载体上,与GFP融合表达,最终可以通过观察GFP的荧光确定PwMYB20的表达部位。结果显示,空载体GFP分布于整个细胞(图8A, B, C),而PwMYB20与GFP的融合蛋白虽然在细胞质中也有微量表达(图8D),但主要分布在细胞核中(图8D, E, F)。这些结果表明PwMYB20是一个主要定位在细胞核中的蛋白。
2.6 PwMYB20在酵母中的转录激活活性
为验证PwMYB20是否作为转录因子发挥作用,构建不同载体并转化酵母。图9显示,转化空载体pBD和pBD-PwMYB20的酵母不能在-Trp-His-Ade缺陷型培养基中正常生长,而转化阳性对照pBD-ANAC092的酵母可以在-Trp-His-Ade缺陷型培养基中正常生长,表明PwMYB20全长没有转录激活活性。为了研究PwMYB20 N端和C端的激活活性,又分别构建了包含PwMYB20 N端和C端的酵母转化载体,结果显示: PwMYB20-133-225具有转录激活活性,而PwMYB20-1-127不具有转录活性(图9)。这些结果表明包含TAD激活域的PwMYB20的C端具有激活活性,而全长没有转录激活活性,可能是由于N端存在转录抑制结构域。

图6 PwMYB20在青杄各组织的相对表达量Fig.6 Expression analysis of PwMYB20 in different tissues of Picea wilsonii利用单因素方差分析进行差异显著性分析,多重比较方法为Duncan(α=0.05)法,不同字母表示差异显著(P<0.05)。内参基因为青杄EF1-α。下同。Single factor analysis of variance is used to analyze the difference, Duncan (α=0.05) test is used as the multiple comparison method, and different letters indicate significant difference (P<0.05). EF1-α is the reference gene.The same below.

图7 不同处理下PwMYB20的相对表达量Fig.7 Expression analysis of PwMYB20 with different treatments内参基因为青杄EF1-α。各处理0 h时相对表达量为“1”。EF1-α is the reference gene. The relative expression of PwMYB20 with different treatments for 0 h is ‘1’.

图8 PwMYB20的亚细胞定位(标尺: 75 μm)Fig.8 Subcellular localization analysis of PwMYB20(Bar=75 μm)A, B, C: 35S驱动GFP在洋葱(Allium cepa)中瞬时表达(A: 空载体GFP荧光; B: 明场下的洋葱表皮细胞; C: A与B的融合图像); D: PwMYB20-GFP在洋葱中瞬时表达; E: DAPI染色; F: GFP与DAPI荧光重叠。A, B, C: Onion (Allium cepa) epidermal cells that are transformed with GFP driven by 35S promoter (A: The GFP fluorescence of empty plasm; B: Onion epidermal cell under bright field; C: The merged picture of A and B); D: Onion epidermal cell that is transformed with PwMYB20-GFP; E: Onion epidermal cell that is stained by DAPI; F: The overlap between GFP and DAPI fluorescence.
3 讨论
植物中的MYB基因最早在玉米中分离出来,被命名为C1(Paz-Areset al., 1987),随后分离鉴定的MYB转录因子越来越多。拟南芥中已经发现198个MYB相关基因,而在水稻、玉米、二倍体粗山羊草(Aegilopstauschii)等植物中发现MYB基因均超过了200个。本试验从青杄中克隆得到PwMYB20基因,通过多序列对比发现,PwMYB20属于R2R3类型的MYB转录因子(图4)。序列比对发现PwMYB20与其他物种的MYBs在N端有较高的相似性,且为R2R3结构域,在C端则拥有比较特异的转录激活域TAD。与拟南芥相比,C端的结构域是青杄、白云杉等物种特有的。R2R3-MYB类蛋白是植物中数目最多的一类MYB蛋白,拟南芥中198个MYB基因,其中编码R2R3-MYB的MYB基因数量高达126,占60%以上(Chenetal., 2006)。
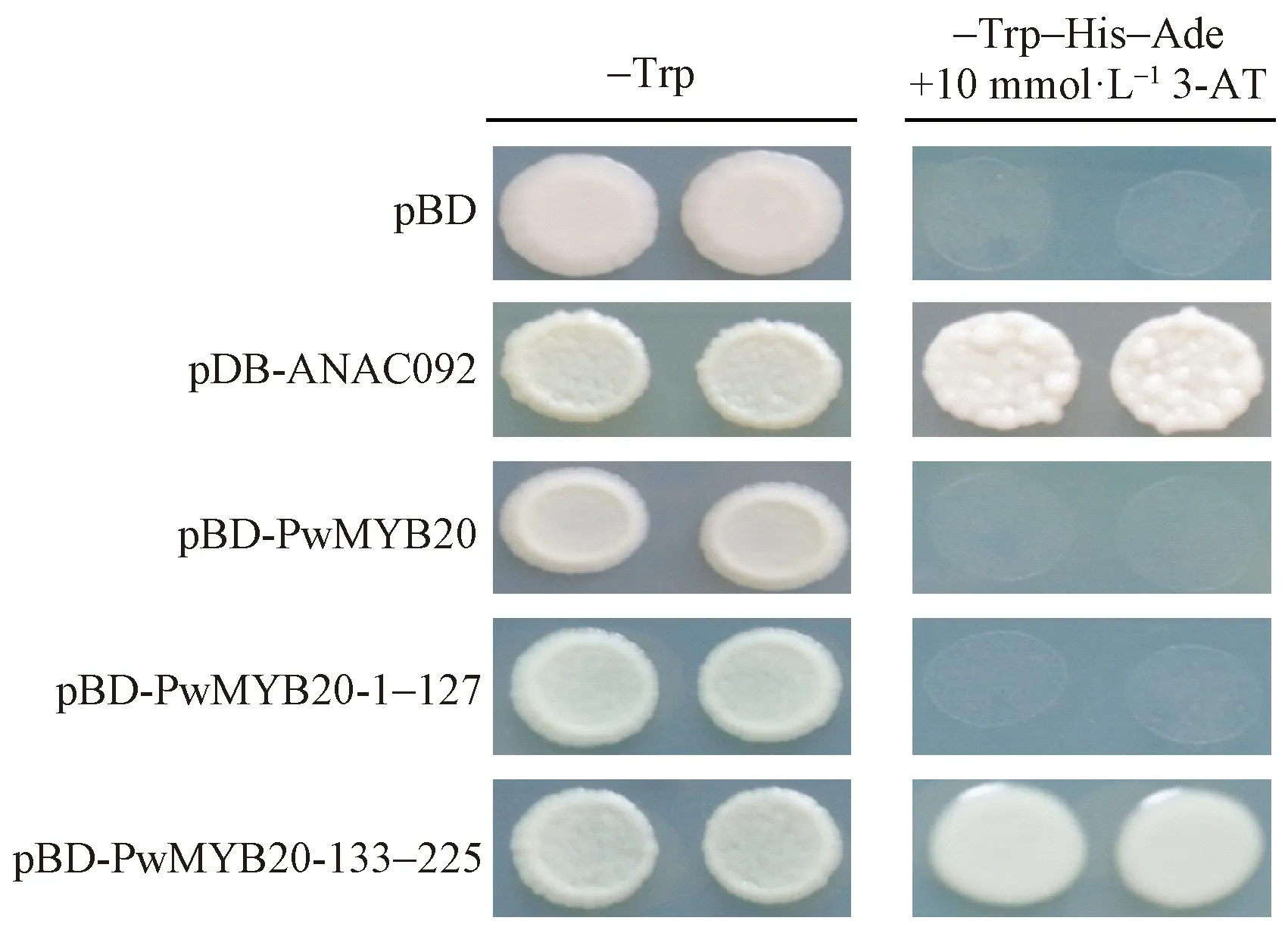
图9 PwMYB20在酵母中的转录激活活性检测Fig.9 Transcriptional activity test of the PwMYB20 proteins in yeast空载体pBD和pBD-ANAC092分别作为阴性和阳性对照,图中蛋白后面的数字指示片段的位置。The pBD vector alone and pBD-ANAC092 are used as negative and positive controls, and the numbers indicate the position of truncated fragments of protein.
本研究通过转录激活活性试验,发现PwMYB20全长及N端没有激活活性,而C端具有激活活性(图9)。Hao等(2010)在研究NAC转录因子的激活活性时发现,NAC的N端存在1个NARD结构域,抑制了NAC的转录激活活性(Haoetal., 2010)。因此,推测PwMYB20 N端可能存在一个转录抑制区域,抑制了PwMYB20的转录激活活性。本文中亚细胞定位试验结果表明,PwMYB20主要存在于细胞核中(图8),此结果与利用WoLF-PSORT工具进行亚细胞定位预测结果相符。因此推测PwMYB20作为一个转录因子主要在细胞核中发挥功能。
以往研究发现MYB转录因子在模式植物及作物类植物响应逆境胁迫中扮演了重要角色(Agarwaletal., 2006; Daietal., 2007; Chenetal., 2013; Baldonietal., 2015)。本文的研究结果显示,PwMYB20在青杄冷胁迫及干旱胁迫后表达量有显著变化(图7),表明其可能在植物冷胁迫及干旱胁迫响应中发挥了功能。在低温处理3 h和12 h时PwMYB20表达量均显著高于未处理幼苗,而在低温处理6 h时PwMYB20表达量与未处理幼苗相比并没有显著变化,表明PwMYB20对于低温处理的响应存在时间上的差异,主要在处理早期和后期发挥作用。类似地,Shi等(2014)研究发现,AtHAP5A和AtXTH21在NaCl处理3,6,24 h时表达量均明显上升,而NaCl处理12 h时表达量却没有明显变化。
根据在植物抗逆胁迫过程中对ABA信号传导途径的依赖性,可将MYB转录因子分成2类: 一类是和ABA信号相关的MYB转录因子,而另一类是独立于ABA信号途径的MYB转录因子。AtMYB60与AtMYB96可以通过ABA信号级联调节气孔运动(Cominellietal., 2005),从而提高抗旱性和抗病性(Seoetal., 2009; 2010)。厚叶旋蒴苣苔(Boeacrassifolia)中的BcMYB1对干旱胁迫响应显著,同时能被低温、PEG、高盐等胁迫诱导,但在外源ABA处理后其表达量却很低,说明可能通过一种不依赖ABA的途径参与调控基因表达从而对逆境产生应答(Chenetal., 2005)。在本试验中,外源ABA处理时PwMYB20表达显著上升(图7),因此推测PwMYB20可能通过ABA途径响应外界非生物胁迫,但其具体的调控与响应机制有待深入研究。
4 结论
青杄PwMYB20,作为一个R2R3类型的MYB转录因子发挥作用,其转录激活活性位于C端。PwMYB20在青杄各组织中均有表达,属于组成型表达。此外,在干旱、低温和ABA处理下PwMYB20表达发生显著变化,说明其普遍参与了青杄应对逆境胁迫的响应过程。
李长江, 崔晓燕, 孙 帆, 等. 2014. 青杄干旱诱导基因PwWDS1的cDNA分离与表达分析. 林业科学, 50(4): 129-136.
(Li C J, Cui X Y, Sun F,etal. 2014. Isolation and expression analysis ofPwWDS1 inPiceawilsonii. Scientia Silvae Sinicae, 50(4): 129-136. [in Chinese])
许家春, 邵海燕, 李殿波. 2004. 优良绿化树种青杄云杉引种栽培技术. 中国林副特产, (3): 24-25.
(Xu J C, Shao H Y, Li D B. 2004. Introduction and cultivation techniques ofPiceawilsonii. Forest By-Product and Speciality in China, (3): 24-25. [in Chinese])
杨映根, 桂耀林, 唐 巍, 等. 1994. 青杄愈伤组织在继代培养中的分化能力及染色体稳定性研究. 植物学报, 36(12): 934-939.
(Yang Y G, Gui Y L, Tang W,etal. 1994. Observation on differentiation potential and chromosome stability of callus in subculture ofPiceawilsonii. Journal of Integrative Plant Biology, 36(12): 934-939. [in Chinese])
张大勇, 赵松岭, 张鹏云, 等. 1989. 青杄林恢复演替过程中的邻体竞争效应及邻体干扰指数的改进模型. 生态学报, 9(1): 53-58.
(Zhang D Y, Zhao S L, Zhang P Y,etal. 1989. An improved model of neighborhood competition effect and neighborhood disturbance index in the process of restoration and succession in spruce forest. Acta Ecologica Sinica, 9(1): 53-58. [in Chinese])
张 盾, 刘亚静, 李长江, 等. 2012. 青杄均一化cDNA文库构建及EST序列分析. 生物技术通报, (6): 71-76.
(Zhang D, Liu Y J, Li C J,etal. 2012. Construction of normalized cDNA library and analysis of corresponding EST sequences inPiceawilsonii. Biotechnology Bulletin, (6): 71-76. [in Chinese])
张 通, 李巧玲, 张凌云. 2014.PwEXP1在青杄种子萌发及逆境响应中的表达特征. 林业科学, 50(12): 56-62.
(Zhang T, Li Q L, Zhang L Y. 2014. Expression characteristics ofPwEXP1 gene in seed germination and adversity inPiceawilsonii. Scientia Silvae Sinicae, 50(12): 56-62. [in Chinese])
Abe H, Urao T, Ito T,etal. 2003.ArabidopsisAtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. The Plant Cell, 15(1): 63-78.
Agarwal M, Hao Y J, Kapoor A,etal. 2006. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. Journal of Biological Chemistry, 281(49): 37636-37645.
Al-Attala M, Wang X, Abou-Attia M,etal. 2014. A novelTaMYB4 transcription factor involved in the defence response againstPucciniastriiformisf. sp.triticiand abiotic stresses. Plant Molecular Biology, 84(4/5): 589-603.
Ambawat S, Sharma P, Yadav N R,etal. 2013. MYB transcription factor genes as regulators for plant responses: an overview. Physiology and Molecular Biology of Plants, 19(3): 307-321.
Baldoni E, Genga A, Cominelli E. 2015. Plant MYB transcription factors: Their role in drought response mechanisms. International Journal of Molecular Sciences, 16(7): 15811-15851.
Broun P. 2004. Transcription factors as tools for metabolic engineering in plants. Current Opinion in Plant Biology, 7(2): 202-209.
Chen B J, Wang Y, Hu Y L,etal. 2005. Cloning and characterization of a drought-inducible MYB gene fromBoeacrassifolia. Plant Science, 168(2): 493-500.
Chen Y, Chen Z L, Kang J Q,etal. 2013.AtMYB14 regulates cold tolerance inArabidopsis. Plant Molecular Biology Reporter, 31(1): 87-97.
Chen Y H, Yang X Y, He K,etal. 2006. The MYB transcription factor superfamily ofArabidopsis: Expression analysis and phylogenetic comparison with the rice MYB family. Plant Molecular Biology, 60(1): 107-124.
Cominelli E, Galbiati M, Vavasseur A,etal. 2005. A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Current Biology, 15(13): 1196-1200.
Dai X Y, Xu Y Y, Ma Q B,etal. 2007. Overexpression of an R1R2R3 MYB gene,OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenicArabidopsis. Plant Physiology, 143(4): 1739-1751.
Dubos C, Stracke R, Grotewold E,etal. 2010. MYB transcription factors inArabidopsis. Trends in Plant Science, 15(10): 573-581.
Fornale S, Shi X H, Chai C L,etal. 2010. ZmMYB31 directly represses maize lignin genes and redirects the phenylpropanoid metabolic flux. Plant Journal, 64(4): 633-644.
Hao Y J, Song Q X, Chen H W,etal. 2010. Plant NAC-type transcription factor proteins contain a NARD domain for repression of transcriptional activation. Planta, 232(5): 1033-1043.
He X J, Mu R L, Cao W H,etal. 2005. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant Journal, 44(6): 903-916.
Jung C, Seo J S, Han S W,etal. 2008. Overexpression ofAtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenicArabidopsis. Plant Physiology, 146(2): 623-635.
Ma Q H, Wang C, Zhu H H. 2011.TaMYB4 cloned from wheat regulates lignin biosynthesis through negatively controlling the transcripts of both cinnamyl alcohol dehydrogenase and cinnamoyl-CoA reductase genes. Biochimie, 93(7): 1179-1186.
Paz-Ares J, Ghosal D, Wienand U,etal. 1987. The regulatory c1 locus ofZeamaysencodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO, 6(12): 3553-3558.
Seo P J, Park C M. 2010. MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis inArabidopsis. New Phytologist, 186(2): 471-483.
Seo P J, Xiang F N, Qiao M,etal. 2009. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response inArabidopsis. Plant Physiology, 151(1): 275-289.
Shi H T, Ye T T, Zhong B,etal. 2014.AtHAP5Amodulates freezing stress resistance inArabidopsisthrough binding to CCAAT motif ofAtXTH21. New Phytologist, 203(2): 554-567.
Shim J S, Jung C, Lee S,etal. 2013. AtMYB44 regulatesWRKY70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling. Plant Journal, 73(3): 483-495.
Stracke R, Ishihara H, Barsch G H A,etal. 2007. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of theArabidopsisthalianaseedling. Plant Journal, 50(4): 660-677.
Stracke R, Werber M, Weisshaar B. 2001. The R2R3-MYB gene family inArabidopsisthaliana. Current Opinion in Plant Biology, 5(5): 447-456.
Tominaga R, Iwata M, Sano R,etal. 2008.ArabidopsisCAPRICE-LIKE MYB 3 (CPL3) controls endoreduplication and flowering development in addition to trichome and root hair formation. Development, 135(7): 1335-1345.
Urao T, Yamaguchi-Shinozaki K, Urao S,etal. 1993. AnArabidopsismyb homolog is induced by dehydration stress and its gene product binds to the conserved MYB recognition sequence. The Plant Cell, 5(11): 1529-1539.
Wang H Y, Wang H L, Shao H B,etal. 2016. Recent advances in utilizing transcription factors to improve plant abiotic stress tolerance by transgenic technology. Frontiers in Plant Science, 7. Doi: 10.3389/fpls.2016.00067
Yang A, Dai X Y, Zhang W H. 2012. A R2R3-type MYB gene,OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. Journal of Experimental Botany, 63(7): 2541-2556.
Yang S W, Jang I C, Henriques R,etal. 2009. FAR-RED ELONGATED HYPOCOTYL1 and FHY1-LIKE associate with theArabidopsistranscription factors LAF1 and HFR1 to transmit phytochrome a signals for inhibition of hypocotyl elongation. The Plant Cell, 21(5): 1341-1359.
Yu Y L, Li Y Z, Huang G X,etal. 2011. PwHAP5, a CCAAT-binding transcription factor, interacts with PwFKBP12 and plays a role in pollen tube growth orientation inPiceawilsonii. Journal of Experimental Botany, 62(14): 4805-4817.
(责任编辑 徐 红)
Cloning and Expression Analysis of MYB Homologous GenePwMYB20 fromPiceawilsonii
You Hanli Yuan Yihang Li Changjiang Zhang Lingyun
(Key Laboratory of Forestry Silviculture and Conservation of Ministry of Education Beijing Forestry University Beijing 100083)
【Objective】 MYB is the largest family of transcription factor in plants, which is widely involved in the regulation of plant life and play an important role in both plant development and growth,and in the regulation of stress resistance. Cloning and analysis of MYB homologous genePwMYB20 inPiceawilsoniiis propitious to explore the function of PwMYB20 in plant growth and development, for the purpose of efficient use of high-qualified genes inPiceawilsonii.【Method】ThePwMYB20 was cloned and verified based on the cDNA library of the EST sequence ofPwMYB20 with RACE-PCR method. ProtParam, ProtScale, FoldIndex and other bioinformatics software were used to analyze and predict the physical and chemical properties of PwMYB20. The homologous proteins were obtained by BLAST online tools, and their comparative analysis and phylogenetic tree analysis were carried out. The tissue specific expression ofPwMYB20 in different tissues,as well as the changes ofPwMYB20 expression with drought, cold, NaCl and ABA treatments were analyzed using real-time quantitative PCR. Furthermore, subcellular localization and transcriptional activation assay were carried out to reveal its biological properties.【Result】The full length ofPwMYB20 cDNA was 966 bp with an open reading frame (ORF) of 675 bp encoding 225 amino acids. ProtParam analysis showed that the protein molecular formula is C1104H1740N340O330S8, molecular weight is 25.3 kDa and isoelectric point is 9.11. Hydrophobicity analysis with Protscale showed that the hydrophobic sites of PwMYB20 were uniformly distributed, suggesting that the protein is hydrophilic. No protein peptide domain was found with SignalP. Furthermore, Protein inherent disorder analysis showed the protein contains many inherently disordered sequences. In addition, TMHMM tools predicted that the protein has no transmembrane domain. BLAST online tools analysis showed thatPwMYB20 belongs to the MYB family gene, which encodes a R2R3-MYB protein. The results of phylogenetic tree analysis showed that PwMYB20 and PgMYB20 were clustered into one cluster. The real-time quantitative PCR analysis indicated thatPwMYB20 expressed constitutively at a high level in seed, followed by needle, and the least was in pollen. The expression ofPwMYB20 displayed responses to drought, cold and ABA treatments, but slightly to NaCl treatment. With drought treatment, the expression ofPwMYB20 was up-regulated at the early stage, and then decreased after 6 h. Additionally, the expression ofPwMYB20 was induced when it was 4 ℃ treated for 3 h, 12 h and with a fluctuation in 6 h, the expression showed an up-down-up trend. Moreover, the expression ofPwMYB20 was induced by ABA continuously. Subcellular localization analysis showed that PwMYB20 was mainly localized in the nucleus. Transcriptional activation analysis revealed that C terminal of PwMYB20 had a transcriptional activation activity, whereas the full-length PwMYB20 and its N terminal had no transcriptional activation activity.【Conclusion】 The results indicated that the expression ofPwMYB20 was constitutive in different tissues, and induced by drought, cold and ABA. In addition, PwMYB20 was located in the nucleus. Its C terminal had a transcriptional activation activity, although its full length is not activated.It is widely involved in responding to different stresses.
Piceawilsonii; MYB transcription factor; gene cloning; stress response; gene expression
10.11707/j.1001-7488.20170504
2016-12-29;
2017-02-21。
转基因生物新品种培育重大专项(2016ZX08009003-002)。
S718.46
A
1001-7488(2017)05-0023-10
* 张凌云为通讯作者。

