二芳基二硫醚与硝基芳烃的反应
李术艳,孙莉娜,沈淑君,程天行,程双华,陈久喜
二芳基二硫醚与硝基芳烃的反应
李术艳1,2,孙莉娜1,2,沈淑君1,2,程天行3,程双华3,陈久喜3
(1漳州职业技术学院食品与生物工程系,福建漳州 363000;2农产品深加工及安全福建省高校应用技术工程中心,福建 漳州 363000;3温州大学化学与材料工程学院,浙江温州 325035)
以二芳基二硫醚(ArSSAr)和硝基芳烃(ArNO2)为原料,在廉价易得的甲醛次硫酸氢钠(Rongalite®)和碳酸钾(K2CO3)共同促进下,以二甲亚砜(DMSO)为溶剂,50℃下合成了一系列非对称二芳基硫醚衍生物,产物结构经1H NMR和13C NMR确证。该方法具有反应条件温和、原料易得和操作简单等优点。
二芳基二硫醚;硝基芳烃;甲醛次硫酸氢钠;二芳基硫醚;反应机理
引 言
二芳基硫醚是一类非常重要的含硫化合物,被广泛应用于药物设计与开发[1-4]。许多含有芳基硫醚官能团的化合物具有多种生物活性。因此,二芳基硫醚衍生物的合成备受关注。早在1984年,Lindley等[5-6]报道了在六甲基磷酰胺等极性溶剂中高温条件下(>200℃)进行芳基卤化物与硫醇铜盐或硫酚(醇)的反应。近年来,过渡金属催化的卤代芳烃与硫酚的偶联反应制备硫醚的策略成为研究热点[7-11]。最近,Bahekara等[12]报道了硝基芳烃与硫酚的偶联反应,实现了C—S的形成反应以构建二芳基硫醚衍生物(图1)。
虽然近年来芳基硫醚的合成取得重要进展[13-30],但这些方法仍存在一些缺点,如使用过量的具有恶臭味液态状的硫醇或硫酚,较长的反应时间,计量甚至过量的不稳定的催化剂,苛刻的反应条件等。因此,开发一种简便的合成方法制备芳基硫醚仍然具有重要意义。
甲醛次硫酸氢钠(Rongalite®),也称雕白粉,是一种非常廉价的还原性试剂,被广泛应用于化工医药中间体的合成[31]。本课题组已报道了雕白粉促进二硫醚的活化,与环氧化合物的开环反应[32]、,-不饱和羰基化合物的Michael反应[33]。而甲醛次硫酸氢钠促进的二硫醚与硝基芳烃的反应至今没有报道。本文使用甲醛次硫酸氢钠为促进剂活化二芳基二硫醚(替代具有恶臭味的硫酚),进而与硝基芳烃通过加成-消除反应制备非对称芳基硫醚衍生物。
1 实验部分
1.1 实验药品
对硝基苯甲醛(分析纯)、对二硝基苯(分析纯)、对硝基苯乙酮(分析纯)、4,4′-二甲基二苯基二硫醚(分析纯)、4,4′-二羟基二苯基二硫醚(分析纯)、二苯基二硫醚(分析纯)均购于百灵威科技有限公司;二甲亚砜(分析纯)、碳酸钾(分析纯)、碳酸钠(分析纯)、碳酸氢钠(分析纯)、碳酸铯(分析纯)、,-二甲基甲酰胺(分析纯)均购于上海阿拉丁生化科技股份有限公司;环己烷(分析纯)、甲苯(分析纯)、甲醇(分析纯)、三乙胺(分析纯)、二水合氟化钾(分析纯)均购于天津市科密欧化学试剂有限公司;薄层层析硅胶板(2.5 cm×8 cm) 购于烟台江友硅胶开发有限公司。
1.2 反应及分析测试仪器
AVANCE-300型或ADVANCE-500型核磁共振仪,TMS为内标,CDCl3为溶剂,Bruker公司;MicrOTOF-QII高分辨质谱,Bruker公司;WRS-1B数字熔点仪,上海精密科学仪器有限公司;ZF-20D型暗箱式紫外分析仪,河南爱博特科技发展有限公司;DSB-2100型旋转蒸发仪,上海爱朗仪器有限公司;智能恒温磁力搅拌器(ZNCL-QS 130-60),河南爱博特科技发展有限公司;TLE104E/02型电子天平,梅特勒-托利多仪器(上海)有限公司。
1.3 非对称二芳基硫醚化合物的合成
在装有磁力搅拌器的干燥的Schlenk管中加入硝基芳烃(0.4 mmol)、二芳基二硫醚(0.3 mmol)、甲醛次硫酸氢钠(0.6 mmol)、K2CO3(0.45 mmol)和DMSO(2 ml),常温搅拌溶解后,升温至50℃充分搅拌3 h。整个实验过程用高效薄层色谱板(TLC)进行监测,反应完毕后,反应液用20ml水洗,然后再用乙醚30 ml分3次萃取,合并有机相,无水硫酸镁干燥,过滤后浓缩,所得的粗产物用0.037~0.048 mm硅胶柱层析分离纯化制得非对称二芳基硫醚。
当使用对硝基苯甲醛作为反应底物时,反应在氮气氛围中进行。
2 实验结果与讨论
2.1 反应条件筛选
选用如图2所示的对硝基苯乙酮(1a)和二苯基二硫醚(2a)作为模板反应,考察不同条件(如溶剂、碱、配比等)对反应结果的影响。
2.1.1 溶剂的筛选 首先考察了溶剂对反应的影响。如表1所示,溶剂对反应的影响较大,当选用环己烷、甲苯或甲醇作为溶剂时,只得到非常少量的目标产物4-(苯硫基)苯甲醛(3a)。然而,当反应在,-二甲基甲酰胺(DMF)中进行时,3a的收率提高至52%。当选用二甲亚砜(DMSO)为溶剂,3a的收率进一步提高至77%。因此,在接下来的反应条件筛选选取DMSO作为溶剂。
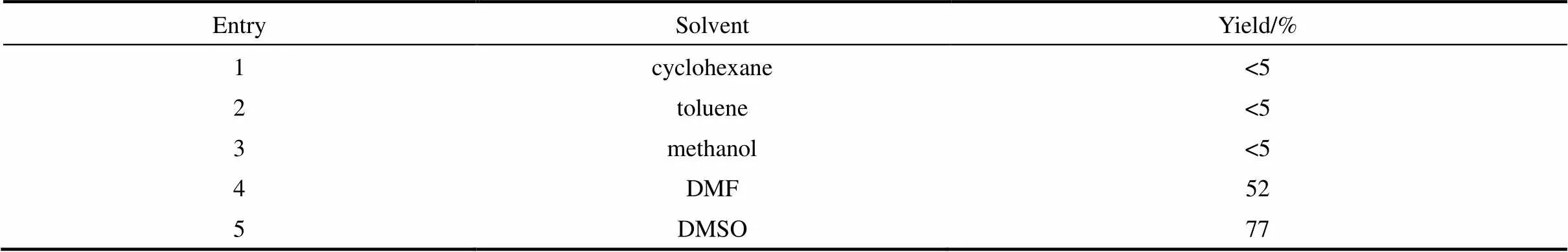
表1 溶剂筛选
Note: Reaction conditions: 1a (0.4 mmol ), 2a (0.3 mmol),K2CO3(0.45 mmol) and Rongalite®(0.6 mmol), solvent (2 ml), 3 h, 50℃.
2.1.2 碱的筛选 如表2所示,碱对该反应也有一定的影响。在所有被考察的碱性化合物中,当选用KF·2H2O作为碱时,反应只得到痕量目标产物3a。
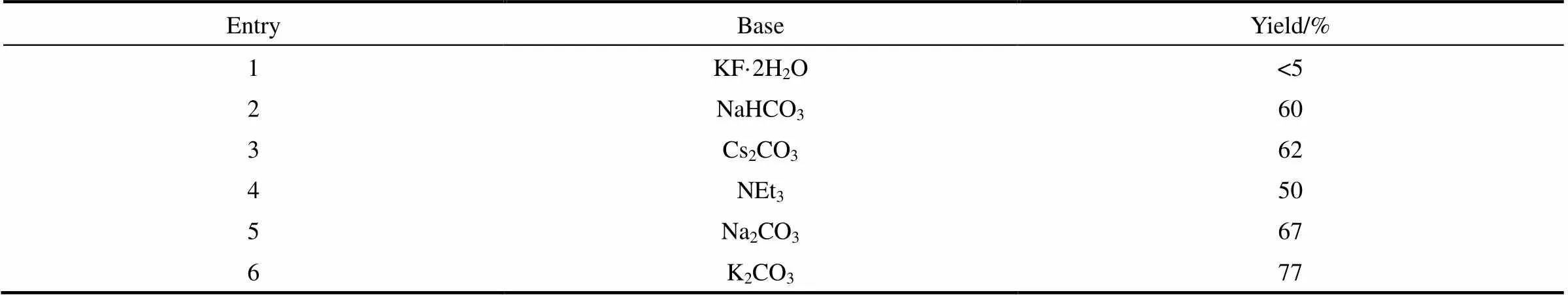
表2 碱筛选
Note: Reaction conditions: 1a (0.4 mmol ), 2a (0.3 mmol),base (0.45 mmol) and Rongalite®(0.6 mmol), DMSO (2 ml), 3 h, 50℃.
当使用其他的无机碱(如NaHCO3、Cs2CO3和Na2CO3)或有机碱(如三乙胺,NEt3),3a的收率分别为60%、62%、67%和50%。以K2CO3作为碱时的收率可达77%。
2.1.3 碱及甲醛次硫酸氢钠的用量对反应的影响
以DMSO为反应溶剂,K2CO3为碱性条件,考察了碱和甲醛次硫酸氢钠(Rongalite®)的用量对反应的影响。由表3可见,碱和甲醛次硫酸氢钠对该反应至关重要,不加两者中任意一种,该反应无法进行。首先筛选K2CO3的用量,当反应体系中加入0.15 mmol的K2CO3时,产物3a的收率为30%;当K2CO3的量增至0.3 mmol,产物3a的收率提高至65%;当K2CO3的量为0.45 mmol时,产物3a的收率最高(77%);继续K2CO3的量增至0.6 mmol时,产率并没有明显的提高。另一方面,通过筛选Rongalite®的用量发现当反应体系中加入0.6 mmol的Rongalite®时,产物3a的收率为77%。
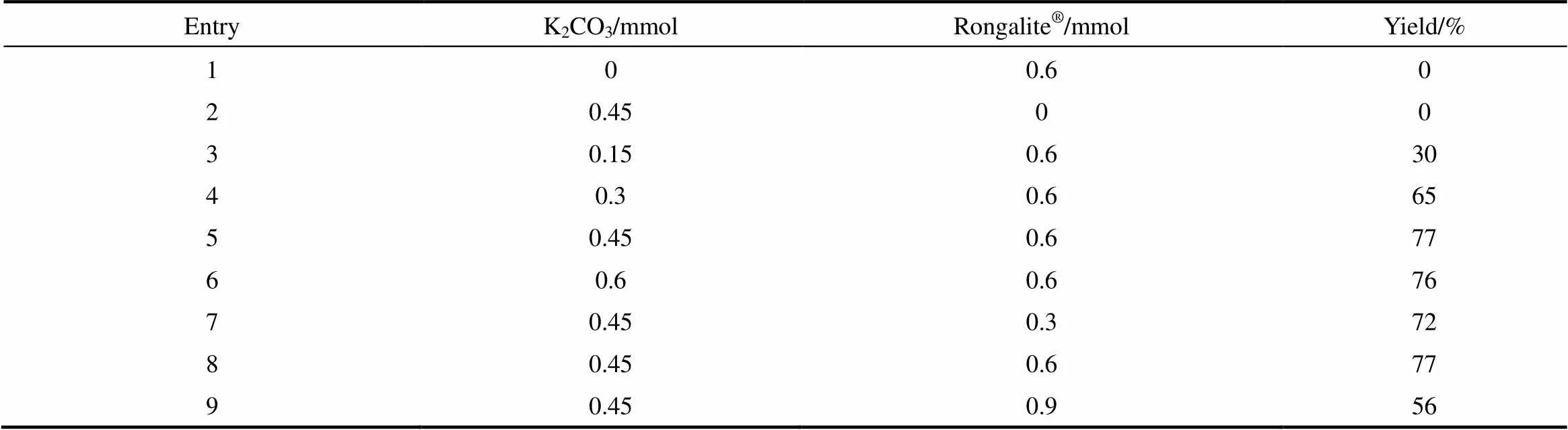
表3 碱及促进剂的用量对反应的影响
Note: Reaction conditions: 1a (0.4 mmol ), 2a (0.3 mmol),DMSO (2 ml), 3 h, 50℃.
2.2 底物范围
通过以上的筛选,获得较佳的反应条件如下:对硝基苯乙酮(0.4 mmol)、二苯基二硫醚(0.3 mmol)、Rongalite®(0.6 mmol),K2CO3(0.45 mmol),DMSO (2 ml),50℃,3 h。为进一步研究该反应条件的普适性,探讨了含其他取代基团硝基芳烃与二芳基二硫醚的反应情况。
如表4所示,对硝基苯乙酮(1a)与二对甲苯基二硫醚(2b)、二对羟基苯基二硫醚(2c)的反应都能平稳地进行,相应的目标产物3b和3c收率分别为61% 和72%。有趣的是,当使用1,4-二硝基苯(1b)为底物时,反应也能顺利进行;如序号4~6所示,选择性地合成了目标产物3d~3f,收率分别是67%、61%和66%。值得一提的是,当使用对硝基苯甲醛(1c)为底物时,采用在N2保护条件下、降低反应温度(25℃)的策略避免对硝基苯甲醛的甲酰基(CHO)被氧化。如序号7~9所示,1c与二苯基二硫醚(2a)、二对甲苯基二硫醚(2b)和二对羟基苯基二硫醚(2c)的反应,所得的目标产物3g~3i的收率分别为79%、70%和73%。当然,该方法也存在底物使用范围窄的局限性。例如,当使用富电子的硝基芳烃作为底物时,该反应无法进行。
表4 底物范围
Table 4 Substrates scope
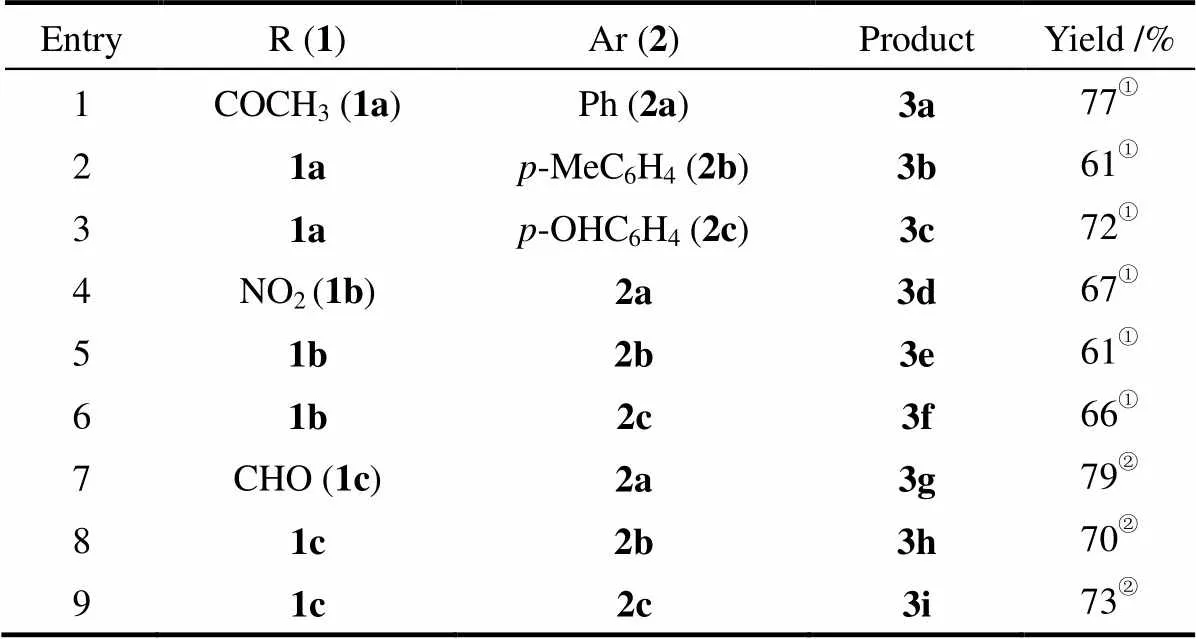
EntryR (1)Ar (2)ProductYield /% 1COCH3 (1a)Ph (2a)3a77① 21ap-MeC6H4 (2b)3b61① 31ap-OHC6H4(2c)3c72① 4NO2 (1b)2a3d67① 51b2b3e61① 61b2c3f66① 7CHO (1c)2a3g79② 81c2b3h70② 91c2c3i73②
① Reaction conditions: 1 (0.4 mmol ), 2 (0.3 mmol), K2CO3(0.45 mmol) and Rongalite®(0.6 mmol), DMSO (2 ml), 3 h, 50℃. ②At 25℃ under N2atmosphere.
2.3 产物结构表征
1-(4-苯硫基)苯乙酮(3a): 熔点: 62~63℃;1H NMR (CDCl3, 300 MHz): 2.55 (s, 3H), 7.83~7.19 (m, 9H);13C NMR (CDCl3, 125 MHz):26.5,127.5, 128.8, 128.9, 129.7, 132.1, 133.9, 134.5, 144.9, 197.1。HRMS (/): [M+H]+calcd. for C14H13OS: 229.0682; found: 229.0677。
1-(对甲苯硫基)苯乙酮(3b): 熔点: 92~93℃;1H NMR (CDCl3, 300 MHz): 2.39 (s, 3H), 2.53 (s, 3H), 7.13~7.23 (m, 4H), 7.39~7.80 (m, 4H);13C NMR (CDCl3, 125 MHz):21.1, 26.3, 126.5, 127.8, 128.7, 130.4, 134.0, 134.3, 139.2, 145.8, 196.9。HRMS (/): [M+H]+calcd. for C15H15OS: 243.0838; found: 243.0835。
1-(对羟基苯硫基)苯乙酮(3c): 油状物;1H NMR (CDCl3, 300 MHz):2.55 (s, 3H), 5.20 (s, 1H), 6.90~7.81 (m, 8H);13C NMR (CDCl3, 125 MHz):25.8, 117.0, 120.8, 125.8, 130.1, 133.1, 137.2, 149.1, 157.2, 191.4。HRMS (/): [M+H]+calcd. for C14H13O2S: 245.0631; found: 245.0638。
4-(对硝基苯基)苯基硫醚(3d): 熔点: 53~54℃;1H NMR (CDCl3, 300 MHz): 7.16~8.08 (m, 9H);13C NMR (CDCl3, 125 MHz):124.1, 126.7, 129.7, 130.1, 130.5, 134.8, 145.4, 148.5。HRMS (/): [M+H]+calcd. for C12H10NO2S: 232.0427; found: 232.0423。
4-(对硝基苯基)(对甲基苯基)硫醚(3e): 熔点: 83~84℃;1H NMR (CDCl3, 300 MHz): 2.42 (s, 3H), 7.12~8.07 (m, 8H);13C NMR (CDCl3, 125 MHz):21.3, 124.0, 126.1, 126.5, 130.9, 135.1, 140.2, 142.2, 149.3。HRMS (/): [M+H]+calcd. for C13H12NO2S: 246.0583; found: 246.0588。
4-(对硝基苯硫基)苯酚(3f): 油状物;1H NMR (CDCl3, 300 MHz): 5.21 (s, 1H), 6.933~7.10 (m, 4H), 7.43~8.06 (m, 4H);13C NMR (CDCl3, 125 MHz):117.2, 120.2, 124.0, 125.6, 137.4, 145.0, 150.1, 157.6。HRMS (/): [M+H]+calcd. for C12H10NO3S: 248.0376; found: 248.0379。
4-(苯硫基)苯甲醛(3g): 熔点: 53~54℃;1H NMR (CDCl3, 300 MHz): 7.23~7.45 (m, 5H), 7.52~7.74 (m, 4H), 9.91 (s, 1H);13C NMR (CDCl3, 125 MHz):127.2, 129.2, 129.8, 130.2, 131.3, 133.7, 134.4, 147.3, 191.3。HRMS (/): [M+H]+calcd. for C13H11OS: 215.0525; found: 215.0529。
4-(对甲基苯硫基)苯甲醛(3h): 熔点: 54~55℃;1H NMR (CDCl3, 300 MHz): 2.41 (s, 3H), 7.17~7.26 (m, 4H), 7.42~7.71 (m, 4H), 9.89 (s, 1H);13C NMR (CDCl3, 125 MHz):21.3, 126.5, 127.2, 130.0, 130.6, 133.4, 134.8, 139.7, 148.1, 191.1。HRMS (/): [M+H]+calcd. for C14H13OS: 229.0682; found: 229.0689。
4-(对羟基苯硫基)苯甲醛(3i): 油状物;1H NMR (CDCl3, 300 MHz): 5.20 (s, 1H), 6.93~7.15 (m, 4H), 7.42~7.71 (m, 4H), 9.88 (s, 1H);13C NMR (CDCl3, 125 MHz):116.9, 125.8, 128.8, 133.8, 137.1, 146.9, 157.0, 196.0。HRMS (/): [M+H]+calcd. for C13H11O2S: 231.0474; found: 231.0479。
3 结 论
本文研究了以廉价的甲醛次硫酸氢钠为反应促进剂,利用低毒无臭而且易于操作的二芳基二硫醚替代硫酚与硝基芳烃的反应,在温和的反应条件下实现了非对称二芳基硫醚的制备。所得产物采用1H NMR和13C NMR进行确证。该方法具有反应条件温和、原料易得和操作简单等优点,为非对称二芳基硫醚衍生物的制备提供了新策略。
References
[1] LIU L P, STELMACH J E, NATARAJAN S R,Sar of 3,4-dihydropyrido[3, 2-]pyrimidone p38 inhibi- tors[J]. Bioorg. Med. Chem. Lett.,2003, 13: 3979-3982.
[2] LIU G, LINK J T, PEI Z,Discovery of novel-arylthio cinnamides as antagonists of leukocyte function-associated antigen-1/intracellular adhesion molecule-1 interaction (1): Identification of an additional binding pocket based on an anilino diaryl sulfide lead[J]. J. Med. Chem.,2000, 43: 4025-4040.
[3] LIU G, HUTH J R, OLEJNICZAK E T,Novel-arylthio cinnamides as antagonists of leukocyte function-associated antigen-1/intracellular adhesion molecule-1 interaction (2): Mechanism of inhibition and structure-based improvement of pharmaceutical properties[J]. J. Med. Chem.,2001, 44: 1202-1210.
[4] DE MARTINO G, EDLER M C, LA REGINA G,New arylthioindoles: potent inhibitors of tubulin polymerization (2): Structure-activity relationships and molecular modeling studies[J]. J. Med. Chem., 2006, 49: 947-954.
[5] LINDLEY J. Copper assisted nucleophilic-substitution of aryl halogen[J].Tetrahedron,1984, 40: 1433.
[6] YAMAMOTO T, SEKINE Y. Condensation of thiophenols with aryl halides using metallic copper as a reactant. Intermediation of cuprous thiophenolates[J].Can. J. Chem.,1984, 62: 1544-1547.
[7] TERUYUKI K, TAKE-AKI M. Metal-catalyzed carbon-sulfur bond formation[J]. Chem. Rev.,2000, 100: 3205-3220.
[8] KAO H L, LEE C F. Efficient copper-catalyzed S-vinylation of thiols with vinyl halides[J]. Org. Lett.,2011, 13: 5204-5207.
[9] GUILARTE V, FERNÁNDEZ-RODRÍGUEZ M A, GARCÍA-GARCÍA P,.A practical, one-pot synthesis of highly substituted thiophenes and benzo[]thiophenes from bromoenynes and-alkynylbromobenzenes[J]. Org. Lett.,2011, 13: 5100-5013.
[10] XU H J, ZHAO Y Q, FENG T,Chan-Lam-type S-arylation of thiols with boronic acids at room temperature[J]. J. Org. Chem.,2012, 77: 2878-2884.
[11] CHEN C K, CHEN Y W, LIN C H,Synthesis of CuO on mesoporous silica and its applications for coupling reactions of thiols with aryl iodides[J]. Chem. Commun.,2010, 46: 282-284.
[12] BAHEKARA S S, SARKATE A P, WADHAI V M,CuI catalyzed C—S bond formation by using nitroarenes[J]. Catal. Commun.,2013, 41: 123-125.
[13] REZAEI N, MOVASSAGH B. Polystyrene resin-supported CuI-cryptand 22 complex: a highly efficient and reusable catalyst for the formation of aryl-sulfur bonds in aqueous media[J]. Tetrahedron Lett.,2016, 57: 1625-1628.
[14] LAI C S, KAO H L, WANG Y J,A general rhodium-catalyzed cross-coupling reaction of thiols with aryl iodides[J]. Tetrahedron Lett.,2012, 53: 4365-4367.
[15] ZHANG P F, YUAN J Y, LI H R,Mesoporous nitrogen-doped carbon for copper-mediated Ullmann-type C-O/-N/-S cross-coupling reactions[J]. RSC Adv.,2013, 3: 1890-1895.
[16] SINGH G, KUMAR A, MALIK S,TBAHS catalyzed coupling reactions of aryl iodides and aryl bromides with thiols under solvent free conditions[J]. Hetero. Lett.,2013, 3: 183-190.
[17] GUZMÁN-PERCÁSTEGUI E, HERNÁNDEZ D J, CASTILLO I. Calix[8]arene nanoreactor for Cu(I)-catalyzed C—S coupling[J]. Chem. Commun.,2016, 52: 3111-3114.
[18] PANDA N, JENA A K, MOHAPATRA S. Heterogeneous magnetic catalyst for S-arylation reactions[J]. Appl. Catal. A—Gen., 2012, 433/434: 258-264.
[19] LAN M T, WU W Y, HUANG S H,. Reusable and efficient CoCl2·6H2O/cationic 2,2'-bipyridyl system-catalyzed S-arylation of aryl halides with thiols in water under air[J]. RSC Adv., 2011, 1: 1751-1755.
[20] MOVASSAGH B, TAKALLOU A, MOBARAKI A. Magnetic nanoparticle-supported Pd(Ⅱ)-cryptand 22 complex: an efficient and reusable heterogeneous precatalyst in the Suzuki-Miyaura coupling and the formation of aryl-sulfur bonds[J].J. Mol. Catal. A: Chem.,2015, 401: 55-65.
[21] FRINDY S, EL KADIB A, LAHCINI M,Copper nanoparticles stabilized in a porous chitosan aerogel as a heterogeneous catalyst for C—S crosscoupling[J]. ChemCatChem,2015, 7: 3307- 3315.
[22] FU W Q, LIU T T, FANG Z X,High activity and stability in the cross-coupling of aryl halides with disulfides over Cu-doped hierarchically porous zeolite ZSM-5[J]. Chem. Commun.,2015, 51: 5890-5893.
[23] ROSTAMI A, ROSTAMI A, GHADERI A. Copper-catalyzed thioetherification reactions of alkyl halides, triphenyltin chloride, and arylboronic acids with nitroarenes in the presence of sulfur sources[J]. J. Org. Chem., 2015, 80: 8694-8704.
[24] MITSUDOME T, TAKAHASHI Y, MIZUGAKI T,Hydrogenation of sulfoxides to sulfides under mild conditions using ruthenium nanoparticle catalysts[J]. Angew. Chem., Int. Ed.,2014, 53: 8348-8351.
[25] SEBASTIAN KRACKL S, COMPANY A, ENTHALER S. Low-valent molybdenum-based dual pre-catalysts for highly efficient catalytic epoxidation of alkenes and deoxygenation of sulfoxides[J]. ChemCatChem,2011, 3: 1186-1192.
[26] JANG Y, KIM K T, JEON H B. Deoxygenation of sulfoxides to sulfides with thionyl chloride and triphenylphosphine: competition with the pummerer reaction[J].J. Org. Chem.,2013, 78: 6328-6331.
[27] ENTHALER S. A straightforward zinc-catalysed reduction of sulfoxides to sulfides, Enthaler, Stephan[J]. Catal. Sci. Technol., 2011, 1: 104-110.
[28] ABBASI M, MOHAMMADIZADEH M R, MORADI Z. Efficient reduction of sulfoxides with NaHSO3catalyzed by I2[J]. Tetrahedron Lett.,2015, 56: 6610-6613.
[29] YOO B W, YU B R, YOON C M. A facile and efficient procedure for the deoxygenation of sulfoxides to sulfides with the HfCl4/Zn system[J].J. Sulfur Chem.,2015, 36: 358-363.
[30] AMIRI K, AMIN ROSTAMI A, SAMADI S,Cu-ZSM5 as reusable catalyst for the one-pot, odorless and ligand-free C—S bond formation[J]. Catal. Comm.,2016, 86: 108-112.
[31] CHEN J X. Rongalite[J]. Synlett, 2012, 23: 157-158.
[32] GUO W X, LV G S, CHEN J X,Rongalite®and base-promoted cleavage of disulfides and subsequent Michael addition to a,b-unsaturated ketones/esters: an odorless synthesis of α,β-sulfido carbonyl compounds[J]. Tetrahedron,2010, 66: 2297-2300.
[33] GUO W X,CHEN J X, WU H Y,.Rongalite®promoted highly regioselective synthesis of β-hydroxy sulfides by ring-opening of epoxides with disulfides[J]. Tetrahedron,2009, 65: 5240-5243.
Reaction of diaryl disulfides with nitroarenes
LI Shuyan1,2, SUN Lina1,2, SHEN Shujun1,2, CHENG Tianxing3, CHENG Shuanghua3, CHEN Jiuxi3
(1Department of Food and Biology Engineering, Zhangzhou Institute of Technology, Zhangzhou 363000, Fujian, China;2The Applied Technical Engineering Center of Further Processing and Safety of Agriculture Products, Higher Education Institution in Fujian Province, Zhangzhou 363000, Fujian, China;3College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, Zhejiang, China)
The cheap and readily available Rongalite®/K2CO3promoted the reaction of diaryl disulfides with nitroarenes in DMSO at 50 ℃, providing a convenient route to the synthesis of unsymmetrica diaryl sulfide. The structures of all products were characterized by1H NMR and13C NMR. This protocol had some distinct advantages of mild conditions, readily available starting materials and simple work-up.
diaryl sulfide; nitroarenes; sodium formaldehyde sulfoxylate; diaryl sulfide; reaction mechanism
10.11949/j.issn.0438-1157.20161755
O 6
A
0438—1157(2017)06—2394—05
程天行。
李术艳(1981—),女,硕士研究生,讲师。
浙江省自然科学基金项目(LY16B020012);福建省教育厅科技计划项目(JA13388)。
2016-12-15收到初稿,2017-03-15收到修改稿。
2016-12-15.
CHENG Tianxing, ctx@wzu.edu.cn
supported by the Natural Science Foundation of Zhejiang Province (LY16B020012) and the Educational Foundation of Fujian Province (JA13388).

