Clinical Utility of Amlodipine/Valsartan Fixed-Dose Combination in the Management of Hypertension in Chinese Patients
Wenbo He, MD, Zhibing Lu, MD and Hong Jiang, MD
1Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan 430060, People’s Republic of China
Introduction
Hypertension is one of the most prevalent cardiovascular diseases in China and affected around 200 million Chinese in 2006 [1], and this population is still growing. More than 2 million cardiovascular deaths in China were attributed to hypertension in 2005 [2]. In spite of the high prevalence, the blood pressure (BP) control rate in China remains low.According to data collected from China’s tertiary A hospitals in 2010, the BP control rate was only 30.6% [1]. As a developing country, economic factors and poor adherence to antihypertensive therapies in Chinese patients are the most important factors for the low BP control rate. Patients always choose traditional monotherapies such as nifedipine tablets, which are cheap but less efficacious.According to available data, at least 75% of patients with hypertension will require combination therapy to achieve BP targets [3]. However, combination therapy means increased costs and quantity of pills,which contribute to clinical inertia and nonadherence to therapies.
Single-pill, fixed-dose combination (SPC) therapy is a new form of antihypertensive drug therapy that appeared in recent years, and simplifies drug intake and reduces costs compared with combination therapy with two pills. The SPC is recommended by many international guidelines and Chinese guidelines for hypertension. The currently available SPC treatments in China include an angiotensin II type 1 receptor blocker (ARB) combined with a calcium channel blocker (CCB) and an ARB or angiotensinconverting enzyme inhibitor (ACEI) combined with a diuretic (dihydrochlorothiazide in most cases).The ARB/CCB combination is probably more effi-cacious than the ARB/diuretic combination, since CCBs have a greater BP-lowering effect than diuretics. The CCB used in combination therapies is mainly amlodipine (Aml), while a variety of ARBs are used [4]. Thus the ARB used in combination therapies is the key element that in fluences clinical outcomes. Because of different molecular characteristics, different ARBs have different efficacy and other effects [5], among which valsartan (Val) shows advantages in BP-lowering efficacy and improvement of clinical outcomes. For example, Val has a greater effect in reducing left ventricular mass index in hypertensive patients compared with losartan [6]. Moreover, it has been indicated that Aml/Val SPC treatment could increase insulin sensitivity [7]. In many clinical trials, the Aml/Val SPC has shown an additive or synergistic BP-lowering effect[4] and good tolerability in adult patients with mildto-moderate hypertension [8–10]. It has been introduced in China in recent years, and is being more and more widely used in patients with hypertension not adequately controlled by monotherapies.
The Therapeutic Efficacy and Tolerability of Aml/Val SPC Treatment in Chinese Patients
The efficacy and tolerability of Aml/Val SPC treatment in Chinese hypertensive patients have been evaluated in four multicenter studies (Table 1). The commonly used dosage for this SPC is 5 mg Aml plus 80 mg Val daily, which has been investigated in three studies. The earliest trial was a phase 3 registry conducted by Ke et al. [11], which evaluated the efficacy of Aml/Val SPC therapy compared with the component monotherapies (Aml, 5 mg daily, or Val, 80 mg or 160 mg daily) in patients with mildto-moderate hypertension. In 2013 Wang et al. [12]compared the efficacy and safety of Aml/Val SPC therapy with the efficacy and safety of the nifedipinegastrointestinal therapeutic system (GITS) in the EXAM study. In 2014 Hu et al. [14] reported the results of the China Status II study, which was an important prospective observational study and provided powerful evidence for the efficacy and safety of Aml/Val SPC therapy in Chinese hypertensive patients in a real-world setting. Moreover, Zhu et al.evaluated the Aml/Val SPC in a higher dosage (5 mg/160 mg daily) in another multicenter study [13],which compared this SPC therapy with monotherapy with Val (160 mg).
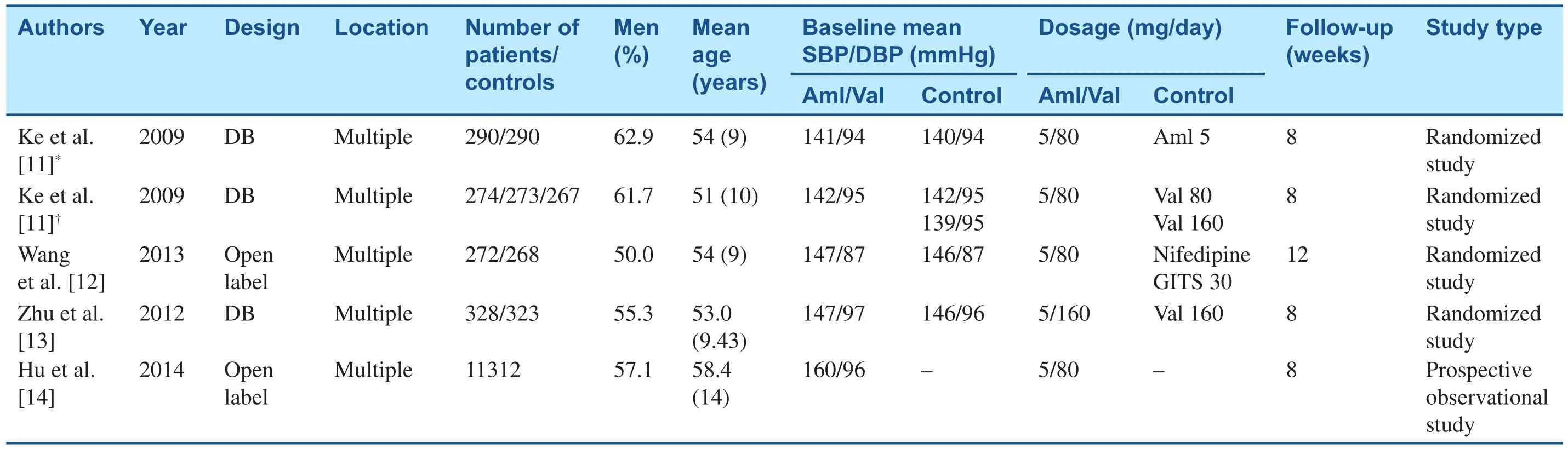
Table 1Characteristics of Clinical Studies on Amlodipine/Valsartan Single-Pill Combination in Chinese Hypertensive Patients.
Evidence from Randomized Trials
The Aml/Val Phase 3 Registry Trial
This trial [11] was a multicenter, randomized, double-blind, active-controlled study, including two parallel group studies. The enrolled patients were aged 18–86 years, with a mean sitting diastolic BP(MSDBP) of 95–110 mmHg. After a washout period of 1–4 weeks, the patients were allocated to one of two parallel studies. In study 1, patients first received Aml (5 mg/day). Then the patients with inadequate BP control (90 mmHg≤MSDBP<110 mmHg) were randomized to receive SPC therapy (Aml/Val,5 mg/80 mg daily) or Aml monotherapy (5 mg/day)for 8 weeks. In study 2, treatment during the first stage was with Val (80 mg/day), and patients were divided into three groups in the second stage(group 1 received Aml/Val, 5 mg/80 mg daily;group 2 received Val, 160 mg/day; group 3 received Val, 80 mg/day). BP was recorded at weeks 2, 4,and 8. The primary efficacy index was the change from the baseline in the mean trough sitting diastolic BP, and the secondary efficacy indices were the change from the baseline in the mean sitting systolic BP (MSSBP), the response rate (the proportion of patients whose MSDBP was less than 90 mmHg or who had a reduction of MSDBP from the baseline of 10 mmHg or more), and the BP control rate (the proportion of patients achieving the BP target, which was 140/90 mmHg for patients with uncomplicated hypertension and 130/80 mmHg for patients with diabetes).
In study 1, 784 patients were enrolled, and the 580 patients who completed the 8-week treatment were included in the primary efficacy analysis. The changes in MSDBP in the SPC group and the Aml group were −10.8 mmHg and −7.8 mmHg respectively, while the changes in MSSBP in these two groups were −12 mmHg and −7.6 mmHg respectively. BP reductions were more evident in the SPC group than in the Aml group (allP<0.0001). The response rate and BP control rate were both significantly higher in the SPC group than in the Aml group (response rate 82.1% vs. 68.3%,P<0.001; BP control rate 71.0% vs. 58.6%,P=0.0014).
In study 2 1021 patients were enrolled, and 814 patients were included in the primary efficacy analysis. The changes in MSDBP in the SPC group, the group that received 80 mg Val daily, and the group that received 160 mg Val daily were −10.8 mmHg,−6.6 mmHg, and −7.6 mmHg respectively, while the changes in MSSBP in the three groups were−12.6 mmHg, −6.2 mmHg, and −7.9 mmHg respectively. BP reductions were more evident in the SPC group than in the Val monotherapy groups (allP<0.0001). The response rate and BP control rate were significantly higher in the SPC group than in the other two groups (response rate 78.1%, 59.7%,and 68.9% respectively; BP control rate 71.2%,46.2%, and 60.7% respectively, allP<0.0001).
Several adverse effects (AEs) of Aml/Val SPC treatment were reported in this trial, among which the commonest AEs were abnormal liver function(2.4%), dizziness (1.0%), hyperlipidemia (1.0%),and peripheral edema (1.5%). The incidence rates of all AEs were 10.7% in the Aml/Val group and 9.0% in the Aml group in study 1. In study 2 the rates were 4.4% in the Aml/Val group, 4.4%, in the group that received 80 mg Val daily, and 4.9% in the group that received 160 mg Val daily. No significant differences in the incidence rates of AEs were found between SPC treatment and Val or Aml monotherapy.
The EXAM Study
The EXAM study [12] was a multicenter, open-label,active-controlled, and parallel group study. This study compared the efficacy and tolerability of Aml/Val SPC therapy with the efficacy and tolerability nifedipine GITS therapy. Nifedipine is considered to one of the most effective antihypertensive agents among CCBs in China, although it is short-acting.The nifedipine GITS, which affords rate-controlled release and once-daily administration, is currently one of the most prescribed antihypertensive agents by Chinese physicians. When hypertension is not adequately controlled by initial monotherapy, many physicians will turn to the nifedipine GITS.
Patients aged 18–65 years from 19 hospitals with hypertension inadequately controlled with initial monotherapy were included. Patients first entered a screening period for 1–2 weeks with existing monotherapies. After that patients with inadequate BP control (140 mmHg≤MSSBP<160 mmHg and/or 90 mmHg≤MSDBP<100 mmHg in the absence of diabetes, 130 mmHg≤MSSBP<160 mmHg and/or 80 mmHg≤MSDBP<100 mmHg for diabetic patients) discontinued their prior therapies and were randomized into an SPC group and a nifedipine GITS group. The SPC group received Aml/Val (5 mg/80 mg daily) and the nifedipine GITS group received the nifedipine GITS (30 mg/ day).Patients were followed up at weeks 2, 4, 8, and 12. The primary efficacy variables were changes in MSDBP and MSSBP from the baseline, and the secondary efficacy variable was the BP control rate.
Five hundred and sixty-four patients were randomized to two groups, and 513 completed the study.After 12 weeks of treatment, the mean reduction in MSSBP in the SPC group was significantly greater than that in the nifedipine GITS group (−16.6 mmHg vs. −10.8 mmHg,P<0.0001). The mean change in MSDBP in the SPC group was greater than that in the nifedipine GITS group (−8.6 mmHg vs.−4.6 mmHg,P<0.0001). A greater proportion of patients had achieved adequate BP control in the SPC group (79.0%) compared with the nifedipine GITS group (57.4%;P<0.0001).
In the EXAM study [12], the top three most frequent AEs were dizziness (3.9%), headache (2.1%),and upper respiratory tract infection (1.8%). The overall incidence rate of AEs in the SPC group was 19.2%, significantly lower than that in the nifedipine GITS group (29.4%,P=0.004). The incidence rate of peripheral edema was lower in the SPC group than in the nifedipine GITS group (0.7% vs.2.8%,P=0.06).
Aml/Val (5 mg/160 mg) Versus Val (160 mg)
In 2012 Zhu et al. [13] reported their study comparing the efficacy and safety of Aml/Val(5 mg/160 mg daily) versus Val (160 mg/day).This was a multicenter, multinational (82.3%were Chinese), double-blind trial on patients with stage 1 or stage 2 hypertension not adequately controlled by Val monotherapy. After a washout period of 1–4 weeks, patients with inadequate BP control (95 mmHg≤MSDBP<110 mmHg)received Val monotherapy (160 mg/day) for 4 weeks. At the end of monotherapy, patients with MSDBP greater than or equal to 95 mmHg but less than 110 mmHg were then randomized to take Aml/Val (5 mg/160 mg daily) or 160 mg Val(160 mg/day) for 8 weeks. The primary efficacy variable was the change from the baseline to week 8 in trough MSDBP, and the secondary efficacy variables included the change from the baseline to week 8 in trough MSSBP, diastolic response rate(proportion of patients with MSDBP of less than 90 mmHg or a reduction in MSDBP of 10 mmHg or more from the baseline), diastolic BP control rate (proportion of patients achieving MSDBP of less than 90 mmHg), and overall BP control rate(proportion of patients achieving mean BP of less than 140/90 mmHg).
Nine hundred and thirty-two patients were enrolled, and 654 participated in the double-blind treatment. Six hundred and twenty- five patients completed the study, and 651 patients were included in the final analysis. Aml/Val SPC therapy resulted in significantly greater mean reductions in MSDBP and MSSBP (−10.3 mmHg and −14.9 mmHg respectively) compared with Val monotherapy(−6.6 mmHg and −7.0 mmHg, respectively) (allP<0.0001). The diastolic response rate was higher in the SPC group (70.1% vs. 52.6%,P<0.0001).A significantly greater proportion of participants achieved BP control in the SPC group than in the Val monotherapy group (diastolic BP control rate 65.9% vs. 50.8%,P<0.0001; overall BP control rate, 61.3% vs. 39.3%,P<0.0001).
The SPC treatment was also well tolerated in this study with the Aml/Val (5 mg/160 mg) SPC [13].Only a few AEs occurred, and all were mild to moderate. The most frequently reported AEs were hyperlipidemia, upper respiratory tract infection,dizziness, hypercholesterolemia, and cough (all with low frequency; ≤1.5%). The incidence rates of all AEs in the SPC group and the Val monotherapy group were 8.8% and 11.7% respectively.
Evidence from an Observational Study
China Status II Study
The China Status II study [14] was a prospective,multicenter, open-label, postmarketing observational study that provided data from the real world on the efficacy and safety of Aml/Val SPC treatment for the first time. Since the patient selection in clinical trials may not accurately reflect patient characteristics in the real world, this study was of great importance to con firm the effects of Aml/Val SPC treatment. A total of 11,422 hypertensive patients with their BP inadequately controlled by monotherapies were included. Patients were prescribed the Aml/Val SPC on the basis of the clinical judgment of the investigators according to the individual patient’s condition. They were treated for 8 weeks and followed up at weeks 4 and 8.The primary efficacy variables were the changes in MSDBP and MSSBP from the baseline, and the secondary efficacy variables were the response rate and BP control rate.
Of 11,422 hypertensive patients, 11,312 were included in the full analysis set, with a mean age of 58.4 years. The duration of hypertension was 8.3±7.3 years, and the baseline MSSBP and MSDBP were 159.6 mmHg and 95.6 mmHg respectively. Among the prior antihypertensive drugs, CCBs were the commonest (47.9%), followed by ARBs (25.2%) and ACEIs (15.3%).After 8 weeks of SPC treatment, MSSBP and MSDBP were significantly reduced by 27.1 mmHg and 15.2 mmHg respectively. When patients were divided into different age groups(<65 years, 65 to <80 years, and ≥80 years), the reductions in MSSBP and MSDBP were all significant, indicating that the efficacy of Aml/Val SPC treatment was independent of age. Patients were also divided into subgroups according to different prior monotherapies or different cardiovascular risk factors, and there were no signifi -cant difference in the BP-lowering effects of the Aml/Val SPC among different subgroups. After 8 weeks of SPC therapy, 76.8% of patients (8692)had achieved their BP targets (<140/90 mmHg)and 98.0% of patients (11,084) responded to treatment (MSSBP reduction of 20 mmHg or more from the baseline or MSDBP reduction of 10 mmHg or more from the baseline).
The incidence rates of AEs with Aml/Val SPC treatment were relatively low in the China Status II study [14], which was a prospective observational study with a large sample size. One hundred and sixty-four cases of AEs were reported (1.4%),among which dizziness (0.2%), headache (0.2%),upper respiratory tract infection (0.2%), and edema(0.2%) were the most frequent. There were only three cases of severe AEs. Further, 86.9% of patients had a high adherence rate (≥80%).
Conclusions
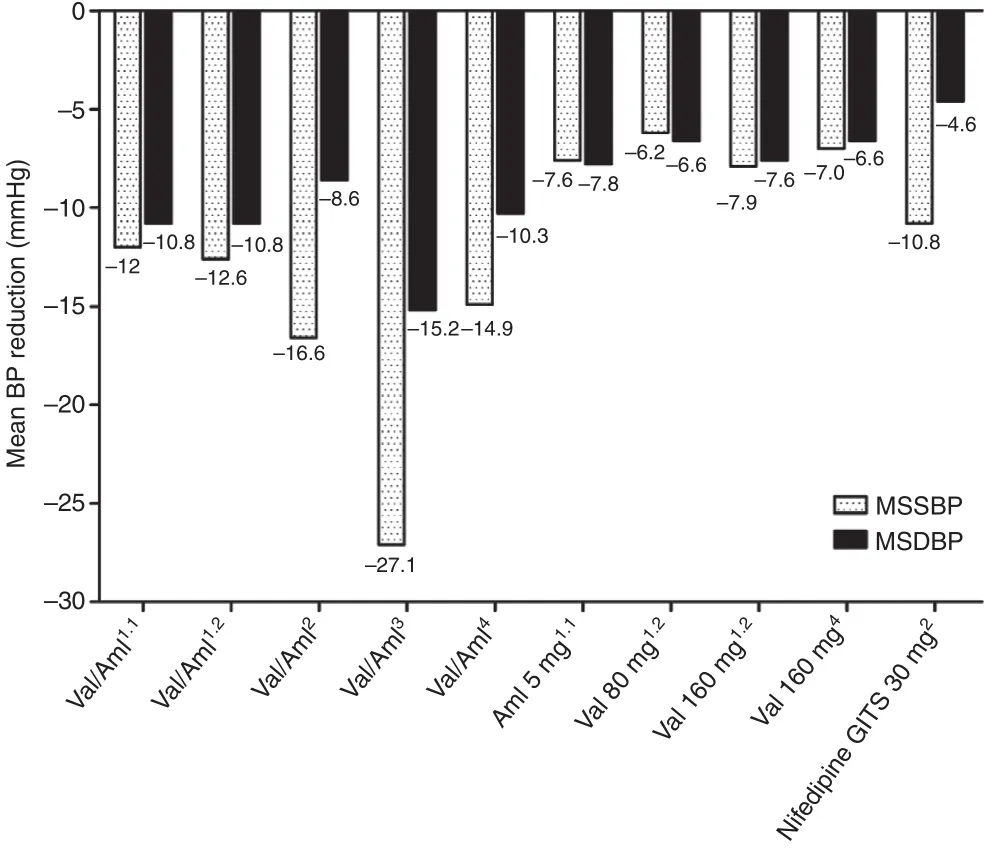
Figure 1 Mean sitting systolic blood pressure (MSSBP)and mean sitting diastolic blood pressure (MSDBP) reductions in different studies. Superscripts 1.1 and 1.2 refer to studies 1 and 2 included in the amlodipine (Aml)/valsartan(Val) single-pill combination phase 3 clinical trial [11],superscript 2 refers to the EXAM study [12], superscript 3 refers to the China Status II study [14], and superscript 4 refers to the study on the Aml/Val (5 mg/160 mg) SPC by Zhu et al. [13]; BP, blood pressure; GITS, gastrointestinal therapeutic system.
Aml/Val SPC therapy showed higher efficacy when compared with Val, Aml, or nifedipine GITS monotherapy. As depicted in Figure 1, according to the aforementioned clinical studies [11, 12,14], Aml/Val (5 mg/80 mg) SPC treatment could reduce MSSBP by 12.0–27.1 mmHg and MSDBP by 8.6–15.2 mmHg. Aml/Val SPC therapy with dosages of 5 mg/160 mg daily showed effects similar to Aml/Val SPC therapy with dosages of 5 mg/80 mg daily. The results were in accordance with the findings of the EX-FAST study conducted by Allemann et al. [15], in which the BP reduction averaged 16.5/9.3 mmHg after 8 weeks of treatment. As reported in the Aml/Val SPC phase 3 registry trial [11], the SPC showed significant advantages in BP-lowering amplitude, BP control rate, and response rate compared with Aml(5 mg/day) and Val (80 mg/day). These results were reasonable since the SPC combined two different drugs, which would increase its antihypertensive efficacy. In addition, the SPC also showed advantages in comparison with a duplicated dose of Val (160 mg/day), which con firmed that the combination of two drugs from different classes was better than simply doubling the dose of either drug, as reported by Wald et al. [16]. Moreover, in the EXAM study [12] Aml/Val SPC was proved to be more efficacious than the nifedipine GITS,which was considered to be one of the most effective antihypertensive agents among CCBs in China. Overall, Aml/Val SPC therapy showed great advantages when compared with monotherapies consisting of commonly used CCBs or ARBs,as revealed in Figure 1. However, there have been no studies comparing the efficacy of Aml/Val SPC with other available SPCs in China.
Several factors may contribute to the improvement in clinical efficacy of Aml/Val SPC treatment. ARBs and CCBs have not only additive but also synergistic influences in their antihypertensive effects. For example, CCBs induce vasodilation and cause BP reduction. This vasodilation could activate the sympathetic nervous system,which influences BP control. When an ARB is used at the same time, sympathetic activation could be diminished by blockage of the reninangiotensin-aldosterone system. Patients’ adherence to drug therapy is another important factor that influences clinical outcomes, especially for a life-long treatment such as antihypertension therapy. Many factors, including drug tolerability,medication frequency or time points, pill burden,and costs, will all contribute to patients’ adherence. SPC treatment will certainly reduce the pill burden, and may help to reduce therapeutic costs in the real world. For instance, combination therapy with Aml tablets (5 mg; ¥5 per tablet) and Val capsules (80 mg; ¥6.4 per capsule) will cost¥11.4 per day in Wuhan. But if Aml/Val tablets(5 mg/80 mg; ¥9 per tablet) are taken, ¥2 will be saved per tablet each day. When multiple drug therapy is indicated, the application of the SPC will make the treatment simpler and cheaper, and in some cases could reduce the rates of AEs, and thus it could improve patients’ adherence.
The Aml/Val SPC was well tolerated in Chinese hypertensive patients. Although several AEs of Aml/Val SPC treatment were reported in the aforementioned clinical studies, the incidence rates of AEs were similar between SPC therapy and Val or Aml monotherapy [11]. In general, the AEs of the SPC reflected the AEs of its component drugs.However, in SPC therapy the overall incidence rates were not significantly increased because of increased drug classes, compared with monotherapies, of the component drugs. Moreover, combination therapy may sometimes help to reduce AEs of the component drugs, because of their different pharmacologic effects. For instance, in the EXAM study [12], the incidence rate of peripheral edema tended to be lower in the Aml/Val group than in the nifedipine GITS group, similar to the results reported by Philipp et al. [9]. Edema is a common AE associated with CCBs, probably caused by increased intracapillary pressure due to attenuated arteriolar constriction [17]. ACEIs and ARBs might alleviate this capillary hypertension by venous dilation, and thus reduce the occurrence of edema [18]. On the other hand, to achieve target BP, monotherapies usually require a higher dose,which will more probably cause side effects [19].In this regard, SPC therapy usually using lower doses of the component drugs has less possibility to produce AEs.
In conclusion, the Aml/Val SPC is highly efficacious and well tolerated in Chinese hypertensive patients. Compared with Val, Aml, or nifedipine GITS monotherapy, it shows greater BP-lowering effects. The Aml/Val SPC could reduce the pill burden, improve patients’ adherence and clinical outcomes, and reduce therapeutic costs, and thus is a cost-effective choice for antihypertensive treatments.
REFERENCES
1. Hu D, Liu L, Yu J, Yao C. National survey of blood pressure control rate in Chinese hypertensive outpatients – China status. Chin J Cardiol 2010;38(3):230–8.
2. He J, Gu D, Chen J, Wu X, Kelly TN, Huang JF, et al. Premature deaths attributable to blood pressure in china: a prospective cohort study.Lancet 2009;374(9703):1765–72.
3. Gradman AH, Basile JN, Carter BL,Bakris GL. Combination therapy in hypertension. J Am Soc Hypertens 2010;4(1):42–50.
4. Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2009). Hypertens Res 2009;32(1):3–107.
5. Miura S, Saku K. Do angiotensin II type 1 receptor blockers have molecular effects? Hypertens Res.2010;33(2):105–6.
6. Picca M, Agozzino F, Pelosi G.Effects of losartan and valsartan on left ventricular hypertrophy and function in essential hypertension.Adv Ther 2004;21(2):76–86.
7. Fogari R, Preti P, Zoppi A,Mugellini A, Corradi L, Lazzari P, et al. Effect of valsartan addition to amlodipine on insulin sensitivity in overweight-obese hypertensive patients. Intern Med 2008;47(21):1851–7.
8. Flack JM, Calhoun DA, Satlin L, Barbier M, Hilkert R, Brunel P. Efficacy and safety of initial combination therapy with amlodipine/valsartan compared with amlodipine monotherapy in black patients with stage 2 hypertension:the EX-STAND study. J Hum Hypertens 2009;23(7):479–89.
9. Philipp T, Smith TR, Glazer R,Wernsing M, Yen J, Jin J, et al. Two multicenter, 8-week, randomized,double-blind, placebo-controlled,parallel-group studies evaluating the efficacy and tolerability of amlodipine and valsartan in combination and as monotherapy in adult patients with mild to moderate essential hypertension. Clin Ther 2007;29(4):563–80.
10. Schunkert H, Glazer RD, Wernsing M, Yen J, Macarie CE, Vintila MM,et al. Efficacy and tolerability of amlodipine/valsartan combination therapy in hypertensive patients not adequately controlled on amlodipine monotherapy. Curr Med Res Opin 2009;25(11):2655–62.
11. Ke Y, Huang J, Zhu J. Efficacy and safety of the single pill combination of valsartan 80 mg plus amlodipine 5 mg in mild to moderate essential hypertensive patients without adequate blood pressure control by monotherapy. Chin J Cardiol 2009;37(9):794–9.
12. Wang JG, Zeng WF, He YS,Chen LL, Wei M, Li ZP, et al.Valsartan/amlodipine compared to nifedipine gits in patients with hypertension inadequately controlled by monotherapy. Adv Ther 2013;30(8):771–83.
13. Zhu D, Yang K, Sun N, Gao P, Wang R, Grosso A, et al.Amlodipine/valsartan 5/160 mg versus valsartan 160 mg in Chinese hypertensives. Int J Cardiol 2013;167(5):2024–30.
14. Hu D, Liu L, Li W. Efficacy and safety of valsartan/amlodipine single-pill combination in 11,422 Chinese patients with hypertension: an observational study. Adv Ther 2014;31(7):762–75.
15. Allemann Y, Fraile B, Lambert M, Barbier M, Ferber P, Izzo JL. Efficacy of the combination of amlodipine and valsartan in patients with hypertension uncontrolled with previous monotherapy: the Exforge in Failure after Single Therapy (EX-FAST) study.J Clin Hypertens (Greenwich)2008;10(3):185–94.
16. Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ.Combination therapy versus monotherapy in reducing blood pressure:meta-analysis on 11,000 participants from 42 trials. Am J Med 2009;122(3):290–300.
17. Pedrinelli R, Dell’Omo G, Mariani M. Calcium channel blockers,postural vasoconstriction and dependent oedema in essential hypertension. J Hum Hypertens 2001;15(7):455–61.
18. Messerli FH, Oparil S, Feng Z.Comparison of efficacy and side effects of combination therapy of angiotensin-converting enzyme inhibitor (benazepril) with calcium antagonist (either nifedipine or amlodipine) versus high-dose calcium antagonist monotherapy for systemic hypertension. Am J Cardiol 2000;86(11):1182–7.
19. Hashmi SK, Afridi MB, Abbas K,Sajwani RA, Saleheen D, Frossard PM, et al. Factors associated with adherence to anti-hypertensive treatment in Pakistan. PLoS One 2007;2(3):e280.
 Cardiovascular Innovations and Applications2017年1期
Cardiovascular Innovations and Applications2017年1期
- Cardiovascular Innovations and Applications的其它文章
- Inherited Cardiomyopathies: Genetics and Clinical Genetic Testing
- The Role of Echocardiography in Hypertrophic Cardiomyopathy
- Rationale and Design of the Randomized Controlled Trial of Intensive Versus Usual ECG Screening for Atrial Fibrillation in Elderly Chinese by an Automated ECG System in Community Health Centers in Shanghai(AF-CATCH)
- The Effect of Home-Based Cardiac Rehabilitation on Functional Capacity,Behavior, and Risk Factors in Patients with Acute Coronary Syndrome in China
- Depression, Anxiety, and Cardiovascular Disease in Chinese: A Review for a Bigger Picture
- Catheter Ablation of Atrial Fibrillation:Where Are We?
