Telocytes involvement in recovery after myocardial infarction
BANCIU Daniel Dumitru,CRETOIU Dragos,CRETOIU Sanda,BANCIU Adela,5(1.Research Beyond Limits SRL,Bucharest 040234,Romania; 2.University of Bucharest,Bucharest 050107,Romania; 3.Carol Davila University of Medicine and Pharmacy,Bucharest 020021,Romania; 4.National Institute of Research-Development in the Pathology Domain and Biomedical Sciences Victor Babes,Bucharest 050096,Romania; 5.Constantin Brancusi University,Gorj 210135,Romania)
·精准与转化医学·
Telocytes involvement in recovery after myocardial infarction
BANCIU Daniel Dumitru1,2,CRETOIU Dragos3,4,CRETOIU Sanda3,4,
BANCIU Adela1,2,5
(1.Research Beyond Limits SRL,Bucharest 040234,Romania; 2.University of Bucharest,Bucharest 050107,Romania; 3.Carol Davila University of Medicine and Pharmacy,Bucharest 020021,Romania; 4.National Institute of Research-Development in the Pathology Domain and Biomedical Sciences Victor Babes,Bucharest 050096,Romania; 5.Constantin Brancusi University,Gorj 210135,Romania)
Myocardial infarction(MI)is a disorder that lowers the lifespan and quality of life.Reperfusion treatment as early as possible is the most effective solution,with an increased focus on post-MI medication.In the recovery process after MI,telocytes(TCs) appear to play an important role,which develops a large number of questions awaiting answers.Defining possible signaling mechanisms involved in recovery after MI may lead to identification the limits of current therapies,and development of new therapeutic solutions.
telocytes;myocardial infarction;stem cell
1 Telocytes and myocardial infarction
Telocytes(TCs),the interstitial cells of mesenchymal origin,have long and thin processes known as telopodes[1]and possesses the ability of responding to specific stimuli.Depending on their localization,TCs present distinct and various characteristics[2-15].At the cardiac level,it has been shown that TCs are distributed as a 3D network,established through adherens junctions,puncta adherentia or stromal synapses[16-17]found mainly in epicardium and at the base and atrial myocardium[18].In myocardial infarction(MI),death of TCs and cardiomyocytes occurs,associated with an impaired regeneration of the affected area[18]. Several studies reveal that TCs’transplantation in the infarction zone leads to improved regeneration post-infarction presumably by stimulating neo-angiogenesis[18-21].
Due to the high importance of cell mobilization in the recovery after MI[22]TCs migration ability and response to various stimuli could lead to their migration and activation into the MI area.TCs response to mechanical stimuli,mediated through voltage-gated calcium channels that have a degree of mechanosensitivity[23],may represent a very important element in thelocal TCs activation by periodic mechanical forces exerted on the myocardium.In the MI affected area,there is a decrease in contractility correlating with evolution of post-MI[24],and local distensions can be assessed as a severity marker for MI[25].
Although TCs have the ability of modulating stem cell responses[26],the number of TCs paradoxically decreases after MI[21].This paradox is increased by the importance of stem cell in post-MI recovery[27].TCs local transplantation in MI promotes increased recovery[18,28].
The first dilemma(D1)outlines the TCs involvement in recovery after MI,but with a decrease in their number.Apparently,in contradiction to this situation,it is observed an increase of cardiac TCs number in exercise-induced cardiac growth[29],which partially confirms the involvement of these cells in cardiac regeneration.
The process of phagocytosis of post-MI necrosis can determine the production of free fatty acids by overexpression of acid ceramidase[30]and therefore,it can lead to a local increase of membrane fluidity that modulates the activation of TCs by mechanical stimuli.
There are evidence indicating that post-MI fibrosis may be inhibited by antagonists of voltage-gated calcium channels,like nifedipine[31]and tetradine[32].But paradoxically,these effects are reported to be independent of cardiomyocytes calcium channels blockers.
An impairment of cardiac fibrosis may lead to cardiac dilation[33].Statins are drugs that modulate cholesterol metabolism and prevent atherosclerosis and MI.Single dose statins before percutaneous coronary intervention may hinder periprocedural MI[34].Statins are involved also in angiogenic cell mobilization after MI[22],and have been intensively used in MI secondary therapy.These are effective in MI secondary therapy[35]but their routine use in this condition is challenged[36].
In this context,the second dilemma(D2)which is represented by the involvement of membrane fluidity in signaling that promotes recovery after MI emerges.On the one hand, the statins generate a decrease in low density lipoprotein(LDL)cholesterol and consequently an increase in membrane fluidity and modulation of sensitivity to mechanical stimuli.Free fatty acids may lead to an increase in membrane fluidity possibly associated with an increase in local fibrosis.On the other hand,blocking voltage-gated calcium channels can promote local fibrosis prevention.
2 Proposed theoretical model
The two dilemmas in understanding the signaling mechanisms of post-MI heart recovery by involving TCs can be unraveled if we assume that there are variables that have not been evaluated in the cited articles.The main variables that may be involved in resolving the dilemmas are the anisotropy of the wound post-MI(V1)and existence of significant changes over time in signaling post-MI(V2).These two sets of variables superimposed on the two dilemmas develop some interesting hypotheses.
The degree of non-uniformity of the lesion post-MI(V1)correlated with the inconsistent number of TCs after MI,even though there is involvement of TCs in the recovery after MI (D1),can be explained by migration of TCs from less affected areas to those intensively damaged by MI and consequently through the existence of experimental biases that are relativelyunlikely.Another possible conclusion(C1)is the real decrease in the number of TCs post-MI with involvement of these cells in the recovery after MI,similar to the behavior of stem cells. This may suggest that TCs have capacity similar to the stem cells,or are a distinct subpopulation of the stem cells(C1).This second hypothesis can justify difficulties in characterizing the support cells for stem cells by their ability of differentiating into stem cells under extreme conditions.
The overlap of changes in signaling post-MI in time(V2)over the degree of inconsistency in the signaling by modulating membrane fluidity(D2)may be explained by the decrease in the number of TCs by converting them into stem cells(C1).In addition to partiall confirmation of C1,it is possible that TCs signaling could be modulated by different mediators according to time elapsed from MI including those as follows:①pH;②various ion concentrations;③other intracytosolic small molecules released quickly after MI;④mediators that slowly change their concentrations,and acts in the late stages after MI,such as those involved in the metabolism of fatty acids(C2).As a logical consequence,because small molecules that are rapidly released after ischemia and post-MI exceed the limits of most affected areas,they can be involved in the recruitment of new TCs or in the increase of local TCs number and late signaling pathways by large molecules may be involved in TCs transformation into stem cell(C3).
The concentration-dependent TCs signaling through MI late stage mediators is schematically shown in Figure 1.
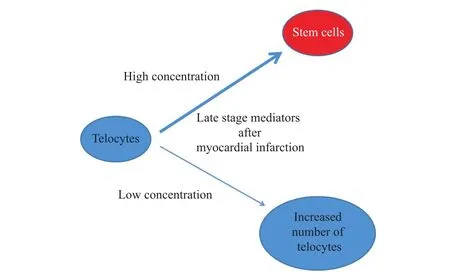
Fig.1 Concentration-dependent effects of late stage mediators after MI,with increased number of TCs or their differentiation into stem cells
3 Conclusions
TCs can be differentiated into stem cells in certain conditions,or presumably subtypes of stem cells.This conclusion may be confirmed by identification of pathways of transforming the cardiac TCs in cells with properties specific for cardiomyocytes.This cellular transformation may be achieved through late stage mediators after MI,possibly by means of metabolic alterations that lead to modulation of ion channels with a degree of mechanosensitivity.Stimulating the number of telocyte in exercise-induced cardiac growth can be associated with lowlevels of mediators required for cellular transformation of TCs in stem cells,suggesting that they have dual functions,stimulating TCs division and differentiation in a concentrationdependent manner.
AcknowledgmentsDaniel Dumitru Banciu and Dragos Cretoiu contributted equally to this work.This work was supported by grants from the Romanian National Authority for Scientific Research,CNCS—UEFISCDI,PNII projects number 82/2012(for Sanda Cretoiu)and 194/2014(for Dragos Cretoiu).
[1]POPESCU L M,FAUSSONE-PELLEGRINI M S.TELOCYTES—a case of serendipity:the winding way from interstitial cells of Cajal(ICC),via interstitial Cajal-like cells(ICLC)to TELOCYTES[J].Journal of Cellular Molecular Medicine,2010,14(4):729-740.
[2]FAUSSONE-PELLEGRINI M S,POPESCU L M.Telocytes[J].Biomolecular Concepts,2011,2(6): 2481-2489.
[3]CRETOIU S M,POPESCU L M.Telocytes revisited[J].Biomolecular Concepts,2014,5(5):353-369.
[4]CRETOIU S M,CRETOIU D,POPESCU L M.Human myometrium-the ultrastructural 3D network of telocytes[J].Journal of Cellular Molecular Medicine,2012,16(11):2844-2849.
[5]XIAO J,WANG F,LIU Z,YANG C.Telocytes in liver:electron microscopic and immunofluorescent evidence[J].Journal of Cellular Molecular Medicine,2013,17(12):1537-1542.
[6]CRETOIU D,CRETOIU S M,SIMIONESCU A A,et al.Telocytes,a distinct type of cell among the stromal cells present in the lamina propria of jejunum[J].Histology and Histopathology,2012, 27(8):1067-1078.
[7]ZHENG Y,LI H,MANOLE C G,et al.Telocytes in trachea and lungs[J].Journal of Cellular and Molecular Medicine,2011,15(10):2262-2268.
[8]POPESCU B O,GHERGHICEANU M,KOSTIN S,et al.Telocytes in meninges and choroid plexus[J].Neuroscience Letters,2012,516(2):265-269.
[9]VANNUCCHI M G,TRAINI C,MANETTI M,et al.Telocytes express PDGFRα in the human gastrointestinal tract[J].Journal of Cellular and Molecular Medicine,2013,17(9):1099-1108.
[10]MOU Y,WANG Y,LI J,et al.Immunohistochemical characterization and functional identification of mammary gland telocytes in the self-assembly of reconstituted breast cancer tissue in vitro[J].Journal of Cellular and Molecular Medicine,2013,17(1):65-75.
[11]KOSTIN S.Myocardial telocytes:a specific new cellular entity[J].Journal of Cellular and Molecular Medicine,2010,14(7):1917-1921.
[12]CANTARERO I,LUESMA M J,JUNQUERA C.The primary cilium of telocytes in the vasculature: electron microscope imaging[J].Journal of Cellular and Molecular Medicine,2011,15(12):2594-2600.
[13]GHERGHICEANU M,POPESCU L M.Cardiomyocyte precursors and telocytes in epicardial stem cell niche:electron microscope images[J].Journal of Cellular and Molecular Medicine,2010, 14(4):871-877.
[14]POPESCU L M,MANOLE E,SERBOIU C S,et al.Identification of telocytes in skeletal muscle interstitium:implication for muscle regeneration[J].Journal of Cellular and Molecular Medicine, 2011,15(6):1379-1392.
[15]CHEN X,ZHENG Y,MANOLE C G,et al.Telocytes in human oesophagus[J].Journal of Cellular and Molecular Medicine,2013,17(11):1506-1512.
[16]CRETOIU D,HUMMEL E,ZIMMERANN H,et al.Human cardiac telocytes:3D imaging by FIBSEM tomography[J].Journal of Cellular and Molecular Medicine,2014,18(11):2157-2164.
[17]POPESCU L M,FERTIG E T,GHERGHICEANU M.Reaching out:junctions between cardiac telocytes and cardiac stem cells in culture[J].Journal of Cellular Molecular Medicine,2016, 20(2):370-380.
[18]LIAO Z F,CAI D Q.Cardiac telocytes in regeneration of myocardium after myocardial infarction[J].Advances in Experimental Medicine and Biology,2016,913:229-239.
[19]MANOLE C G,CISMAGIU V,GHERGHICEANU M,et al.Experimental acute myocardial infarction: telocytes involvement in neo-angiogenesis[J].Journal of Cellular Molecular Medicine,2011, 15(11):2284-2296.
[20]ZHAO B,CHEN S,LIU J,et al.Cardiac telocytes were decreased during myocardial infarction and their therapeutic effects for ischaemic heart in rat[J].Journal of Cellular and Molecular Medicine,2013,17(1):123-133.
[21]ZHAO B,LIAO Z,CHEN S,et al.Intramyocardial transplantation of cardiac telocytes decreases myocardial infarction and improves post-infarcted cardiac function in rats[J].Journal of Cellular Molecular Medicine,2014,18(5):780-789.
[22]HONG S J,CHOI S C,KIM J S,et al.Low-dose versus moderate-dose atorvastatin after acute myocardial infarction:8-month effects on coronary flow reserve and angiogenic cell mobilisation[J].Heart,2010,96(10):756-764.
[23]CAMPEANU R A,RADU B M,CRETOIU S M,et al.Near-infrared low-level laser stimulation of telocytes from human myometrium[J].Lasers in Medical Science,2014,29(6):1867-1874.
[24]WHITE H D,NORRIS R M,BROWN M A,et al.Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction[J].Circulation,1987,76(1): 44-51.
[25]JOYCE E,HOOGSLAG G E,LEONG D P,et al.Association between left ventricular global longitudinal strain and adverse left ventricular dilatation after ST-segment-elevation myocardial infarction[J].Circulation Cardiovascular Imaging,2014,7(1):74-81.
[26]CRETOIU D,RADU B M,BANCIU A,et al.Telocytes heterogeneity:from cellular morphology to functional evidence[J].Seminars in Cell&Developmental Biology,2017(64):26-39.
[27]SCHACHINGER V,ASSMUS B,BRITTEN M B,et al.Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction:final one-year results of the TOPCAREAMI Trial[J].Journal of American College of Cardiology,2004,44(8):1690-1699.
[28]FU S Y,ZHU H,LI S Y,et al.Telocytes in cardiac protection[J].Current Stem Cell Research& Therapy,2016,11(5):390-394.
[29]XIAO J,CHEN P,QU Y,et al.Telocytes in exercise-induced cardiac growth[J].Journal of Cellular Moecular Medicine,2016,20(5):973-979.
[30]SCHULZE H,SCHEPERS U,SANDHOFF K.Overexpression and mass spectrometry analysis of mature human acid ceramidase[J].Biological Chemistry,2007,388(12):1333-1343.
[31]JIA Y,XU J,YU Y,et al.Nifedipine inhibits angiotensinⅡ-induced cardiac fibrosis via downregulating Nox4-derived ROS generation and suppressing ERK1/2,JNK signaling pathways[J]. Pharmazie,2013,68(6):435-441.
[32]TENG G Q,SVYSTONYUK D,MEWHORT H E M,et al.Tetrandrine reverses human cardiac myofibroblast activation and myocardial fibrosis[J].American Journal of Physiology:Heart and Circulatory Physiology,2015,308(12):1564-1574.
[33]NOPPE G,DUFEYS C,BUCHLIN P,et al.Reduced scar maturation and contractility lead to exaggerated left ventricular dilation after myocardial infarction in mice lacking AMPKα1[J]. Journal of Molecular and Cellular Cardiology,2014,74:32-43.
[34]BRIGUORI C,VISCONTI G,FOCACCIO A,et al.Novel approaches for preventing or limiting events(Naples)Ⅱtrial:impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction[J].Journal of the American College of Cardiology,2009,54(23):2157-2163.
[35]TESHIMA Y,YUFU K,AKIOKA H,et al.Early atorvastatin therapy improves cardiac function in patients with acute myocardial infarction[J].Journal of Cardiology,2009,53(1):58-64.
[36]PEDERSEN T R,FAERGEMAN O,KASTELEIN J J P,et al.High-dose atorvastatin vs usualdose simvastatin for secondary prevention after myocardial infarction—the IDEAL study:a randomized controlled trial[J].JAMA,2005,294(19):2437-2445.
10.3969/j.issn.1007-2861.2017.02.004
1007-2861(2017)02-0155-06
Received:Apr.5,2017
Project supported by the Romanian National Authority for Scientific Research,CNCS—UEFISCDI,PNII (82/2012,194/2014)
Banciu Adela,E-mail:adela.banciu79@gmail.com,adela@researchbeyondlimits.ro
Chinese library classification:R 54Document code:A

