A synthetic biological secondary metabolite,LycogenTM,produced and extracted from Rhodobacter sphaeroides WL-APD911 in an optimizatioal scale-up strategy
Cheng-Chin Wang,Shi-Ying Huang,Shu-Hung Huang,Zhi-Hong Wen,Jyun-Ying Huang,Wen-Sheng Liu,Hui-Min David Wang
a Research and Development Department,Chia-Yi Steel,Tainan 717,Taiwan,China
b College of Oceanology and Food Science,Quanzhou Normal University,Quanzhou 362000,China
c Fujian Province Key Laboratory for the Development of Bioactive Material from Marine Algae,Quanzhou 362000,China
d Division of Plastic Surgery,Department of Surgery,Kaohsiung Medical University Hospital,Kaohsiung Medical University,Kaohsiung 807,Taiwan,China
e Graduate Institute of Medicine,College of Medicine,Kaohsiung Medical University,Kaohsiung 807,Taiwan,China
f Center for Stem Cell Research,Kaohsiung Medical University,Kaohsiung 807,Taiwan,China
g Department of Surgery,Faculty of Medicine,College of Medicine,Kaohsiung Medical University,Kaohsiung 807,Taiwan,China
h Orthopaedic Research Center,Kaohsiung Medical University,Kaohsiung 807,Taiwan,China
i Doctoral Degree Program in Marine Biotechnology,National Sun Yat-sen University and Academia Sinica,Kaohsiung 804,Taiwan,China
j Marine Biomedical Laboratory and Center for Translational Biopharmaceuticals,Department of Marine Biotechnology and Resources,National Sun Yat-sen University,Kaohsiung 804,Taiwan,China
k Graduate Institute of Biomedical Engineering,National Chung Hsing University,Taichung 402,Taiwan,China
lDepartment and Graduate Institute of Aquaculture,National Kaohsiung Marine University,Kaohsiung 811,Taiwan,China
m Asia-Pacific Biotech Developing,Inc.Kaohsiung 803,Taiwan,China
n Graduate Institute of Medicine,College of Medicine,Kaohsiung Medical University,Kaohsiung 807,Taiwan,China
o Department of Medical Research,China Medical University Hospital,China Medical University,Taichung 404,Taiwan,China
Abstract The optimization of fermentation medium is important for synthetic biological secondary metabolite productions.The effect of rotation speed,inoculum amount,and medium supplements on the cell growth and LycogenTM secretion of photobacterium Rhodobacter sphaeroides WL-APD911 was evaluated.The results reveal that a higher rotational speed exhibit a higher cell density,and the increasing in the amount ofinoculum amount show a slight augment on the growth of R.sphaeroides WL-APD911.In the case of nitrogen sources adding,LycogenTM production was achieved with a 0.5 mM L-lysine supplementation.Moreover,the attention of Tween 80 presented a tremendous increase in the secondary metabolite.Response surface methodology(RSM)exhibited the optimization of medium supplements for LycogenTM invention is accomplished at molasses concentration of 10 g/L,yeast extract concentration of 40 g/L,0.3% Tween 80 and NaCl concentration of 5 g/L,respectively.Further,the batch fermentation is carried out in both 5 L and 20 L fermentors to study the scale-up process factors to be adopted.At a 20 L fermentor,LycogenTM yields under the optimal culture condition are over 2 times than in the shake flask The present results provide the LycogenTM optimal culture mediums,scale-up procedures and efficien extractions from R.sphaeroides WL-APD911.
Keywords:Rhodobacter sphaeroides WL-APD911;LycogenTM;Response surface methodology(RSM);Ferementation
1.Introduction
Carotenoids are natural hydrophobic lipid-soluble pigments that can have an array of multiple colors,such as yellow,orange or red.They are formed by plants and other photosynthetic microorganism,including bacteria and fungi.Carotenoids in autotrophs absorb sunlight in photosynthetic bio-molecules to prevent the chlorophyll from being damaged by photons.Because the unsubstituted β-ionone rings inner structure contains α-carotene,β-carotene,β-cryptoxanthin and γ-carotene,carotenoids can be converted into retinol,which act as antioxidants when they are consumed by organisms[1].Nearly 90%of carotenoids in the diet and the human body are α- and βcarotene,lycopene,lutein and cryptoxanthin.There are more than 600 types of known carotenoids,with different structures and amounts of unsaturated hydrocarbons.Unsaturated hydrocarbons can combine with free radicals,but carotenoids are used as food colorings,animal feed supplements and have therapeutic and cosmetic applications as anti-oxidative agents and in drugs for chronic diseases.Carotenoids play an important role in the prevention of a range of cancer types and are used in the treatment oflifestyle-related diseases and cardiovascular diseases[2,3].
Due to increasing health consciousness and the demand for carotenoids,the biochemical synthesis ofinatural products that are isolated from plants and microorganisms has become a major focus for study[4].Rhodobacter sphaeroidesis a proteobacteria of the α-3 subclass and its morphology is that of a gramnegative,purple,rod-shaped,non-sulfurous photoheterotrophic bacterium.This species of photosynthetic bacteria contains diverse substances,such as bacteriochlorophylls,biopolymers,5-aminolevulinic acid,carotenoids and coenzyme Q10 [5].It has been shown thatR.sphaeroideshave potential applications in the pharmaceutical and food industries.Recently,the authors generated transformantR.sphaeroidesWL-APD911 from bacteriochlorophylls,which secretes carotenoids such as LycogenTM[6,7].The firs step in generating carotenoids is the condensation of two molecules of geranylgeranyl pyrophosphate to produce phytoene.This is followed by de-saturation to transform the phytoene into colored carotenoids,using phytoene desaturase(CrtI).
Growth studies have been conducted to determine the potential bioactivity of LycogenTMas an anti-inflammator [8,9],or anti-agingagent[10],orforthetreatmentofglucosehomeostasis[11].Many technologies have been used to increase LycogenTMproduction,including optimizing the cultivation conditions,medium supplements,biochemical engineering,metabolic engineering,bio-stimulation and bio-separation[12-14].Response surface methodology(RSM)is a method that combines technical and statistical data for a series of experiments.It was devised by Box and Wilson in 1951 and determines the effect ofindividual variables and their interactions to formulate and develop new products.The experiments in this study use RSM.This study increases the yield ofR.sphaeroidesgrowth and carotenoids during manufacture.The fermentation ofR.sphaeroidesis an expanding industry so industrial production and purificatio is an important consideration.This study determines the optimal conditions for each experimental factor.Because considering one factor at a time is inefficient statistical methods of experimental design are used to determine the interactions between factors and to predict their optimal combinations.
2.Materials and methods
2.1.Chemicals
R.sphaeroidesWL-APD911 was secreted from mutants using chemical mutagenesis and stored in the Bioresource Collection and Research Center (BCRC),Hsinchu,Taiwan.The strain was grown at 30°C on an agar plate of Luria-Bertani(LB)medium.Dulbecco’s modifie eagle’s medium(DMEM),fetal bovine serum(FBS),penicillin and streptomycin were obtained from Gibco BRL company (Grand Island,NY,U.S.A.).Polyclonalanti-iNOSwaspurchasedfromBDBiosciences.NTGand lipopolysaccharide (LPS) were obtained from Sigma-Aldrich Chemical Corporation(St.Louis,MO,USA).
2.2.Culturing of R.sphaeroides WL-APD911
R.sphaeroidesWL-APD911 were inoculated to a pre-culture medium in a 125 mL flas and grown at 30°C and 200 rpm[8,9].After incubation for 3 days,the cells were suspended again and cultured to the main incubation medium at 30°C and 200 rpm for another 3 days,using 1%inoculation in a 250 mL flask
2.3.The optimization of the composition of the media for LycogenTM from R.sphaeroides WL-APD911
The various media components,such as carbon sources(glucose,sucrose,fructose,succinate,molasses and lactose at 5 g/L),nitrogen sources (L-asparagine,glycine,L-glutamine andLlysine at 0.5,1,5,10,15 and 20 mM) and surfactants (Tween 20 and Tween 80 at 0.2%,0.4%,0.6%,0.8% and 1.0%) were supplemented into the production medium to determine the optimal nutrient medium for LycogenTMproduction.An appropriate nutritional control(modifie LB medium)was also maintained.Flasks containing 100 mL ofliquid media with various supplements were incubated in the dark at 30°C on a rotary shaker at 200 rpm for 3 days.
2.4.Bioreactor batch-fed fermentation
The batch fermentation ofR.sphaeroidesWL-APD911 used both 5.0 L and 20.0 L bioreactors and optimal conditions for the culture of the medium(a molasses concentration of 10 g/L,a yeast extract concentration of 40 g/L,0.3%Tween 80 and a NaCl concentration of 5 g/L).The cells were incubated at 30°C for 72 h and agitation was maintained at 200 rpm to ensure homogeneous conditions.During the cultivation period,the cell density and LycogenTMcontent were measured.

Fig.1.Effects of the rotation speed and the inoculum amount on the cell density of R.sphaeroides WL-APD911 in the modifie LB medium.After various treatment for three days,the cell density is measured by the cell dry weight assay according to Materials and Methods.Data are presented as means±SD of three independent experiments.*p <0.05.
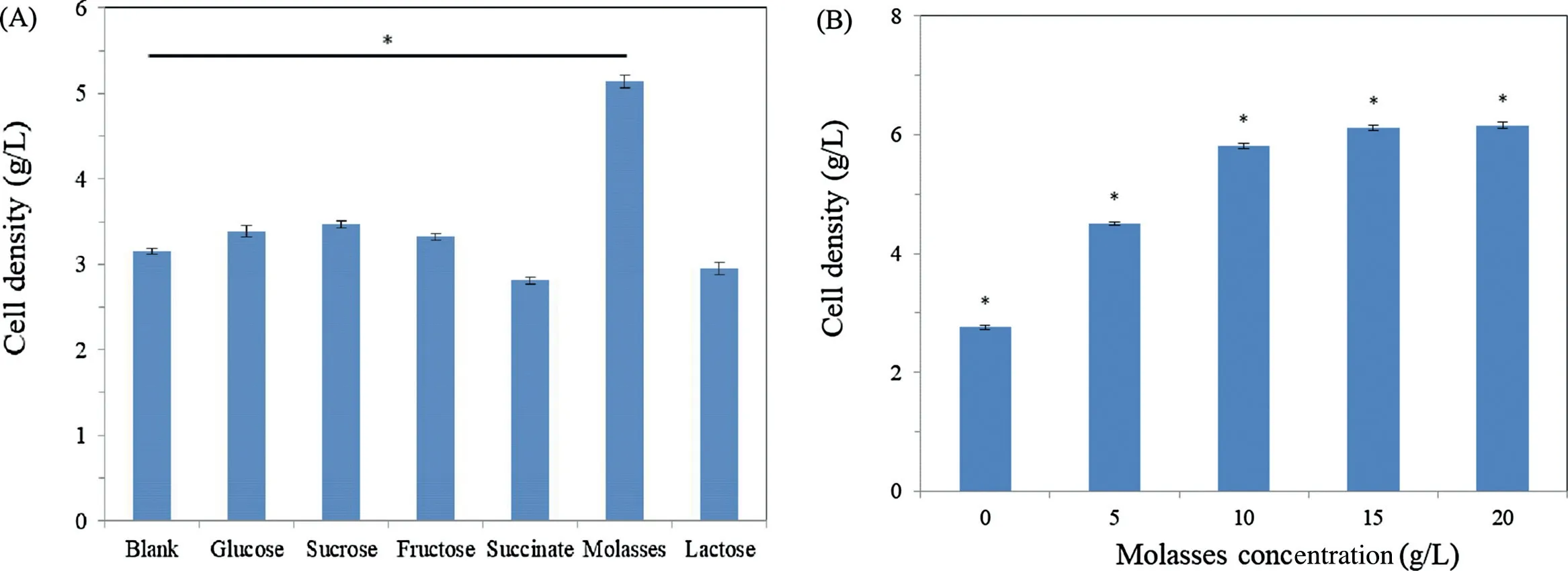
Fig.2.Effects of various carbon sources and molasse concentrations on the cell growth of R.sphaeroides WL-APD911.The baterial cell dry weight is examined according to Materials and Methods.Data are presented as means±SD of three independent experiments.*p <0.05.
2.5.Assay of dry bacteria weight
10 mL liquor of the bacteria broth was centrifuged with 9000 rpm for 5 min and the suspension was then removed.10 mL distilled water was then added well mixed in a centrifuge at 9000 rpm for 5 min for another round[11].The suspension was then removed and the wet bacterial mud was dried at 50°C for the analysis of cell weight.
2.6.Experimental design and optimization using response surface methodology
RSM was used for a set of experimental designs,in order to optimize the contents of the constituents for LycogenTMproduction.The independent variables for the medium components were X1(molasses),X2(yeast extract),X3(Tween 80)and X4(NaCl).These were define as the total concentrations of the minerals in the medium and the proportion of each component was maintained.
2.7.The extraction process for LycogenTM
After harvesting,the bacterial broth was centrifuged at 7500 rpm for 3 min and then washed with ethanol solvent [7].The bacterial residue was extracted using acetone and centrifuged at 7500 rpm for 5 min.The supernatant was filtere using 0.2-μm filte paper.Acetone was completely removed by heating in an oven at 55°C.The extract ofR.sphaeroidesWL-APD911 was named LycogenTM.
2.8.Statistical analysis
All experiments were conducted in triplicate and an analysis of variance(ANOVA)was used to determine the significan variations between the test groups.A value ofp <0.05 was considered to be statistically significant
3.Results and discussion
3.1.The optimization of R.sphaeroides WL-APD91 culture conditions
The modifie LB medium contained 10 g/L tryptone,20 g/L yeast extract.10 g/L NaCl was used to cultureR.sphaeroidesWL-APD91.Fig.1 shows the effect of the rotational speed and the amount ofinoculum on cell growth in the modifie medium.The cell density ofR.sphaeroidesWL-APD911 increases when the rotational speed increases.It has been found that a slower rotational speed produces a lower concentration of dissolved oxygen and can hinder the cell growth [15].The highest cell density is achieved at 200 rpm,which is recommended as the optimal culture condition for cell growth.Fig.1(B)shows that an increase in the amount ofinoculum produces a slight increase in the growth.This shows that 1%inoculum is sufficien to growR.sphaeroidesWL-APD911 in a modifie LB medium.
3.2.The effect ofincubated mediums on cell growth and LycogenTM production
The effect of the addition of glucose,sucrose,fructose,succinate,molasses and lactose on the cell growth ofR.sphaeroidesWL-APD911 is shown in Fig.2(A).It is seen that carbohydrates are necessary for optimal microbial growth and the synthesis of secondary metabolites.The addition of glucose,sucrose,fructose and molasses produces a higher cell density than the addition of succinate or lactose.In a study of the metabolic networks ofR.sphaeroides,it was shown that carbon sources such asD-glucose produce of NADPH in their metabolism via enzymes such as glucose-6-phosphate dehydrogenase and support photosynthetic growth inR.sphaeroidescells.Carbon sources such as succinate do not support photosynthetic growth because the NAD(P)transhydrogenase subunit α(PntAB)activity must be reinforced when using these carbon sources[16].It is worthy of note that the addition of molasses into the culture medium results in the highest cell density of these carbon sources.It has been shown that molasses causes an increase in the biomass and subsequent coenzyme Q10 production byR.sphaeroides[17].As shown in Fig.2(B),the cell density increases as the concentration of molasses increases and is almost saturated at about 10 g/L.It has been shown that molasses significantl promotesR.sphaeroidesgrowth[18].
3.3.The effect of the addition of L-asparagine,glycine,L-glutamine and L-lysine on cell growth and LycogenTM secretion by R.sphaeroides WL-APD911
The addition of 1.0 mML-asparagine gives the maximum cell density (Fig.3),however,the integral area of the LycogenTMis significantl less than in the control.There is also a further increase in the concentration ofL-asparagine,compared to nontreated control vehicle group.When glycine is added,the cell density firstl increases upon the addition of 1.0 mM glycine and then decreases when the concentration of glycine is increased.This demonstrates that increasing the amount of glycine leads to the inhibition of cell growth.The integral area of LycogenTMis also less than that for the control group.The addition ofLasparagine produces a similar result.The data shows that the presences of these additional amino acids have a consequential role in cell growth and LycogenTMproduction.It has been demonstrated that organic nitrogen source is a key factor for bacterial growth and the synthesis of LycogenTM[19].
3.4.The effect of polysorbate surfactants on cell growth and the production of LycogenTM by R.sphaeroides WL-APD911
The presence of Tween 80 in the culture medium slightly inhibits cell growth,especially for the addition of 0.6%Tween 80.The presence of Tween 20 has no significan effect on cell growth(Fig.4).It has been shown that an increase in the quantity of Tween 80 in MRS increases LycogenTMproduction byEnterococcus faeciumST311LD[20].
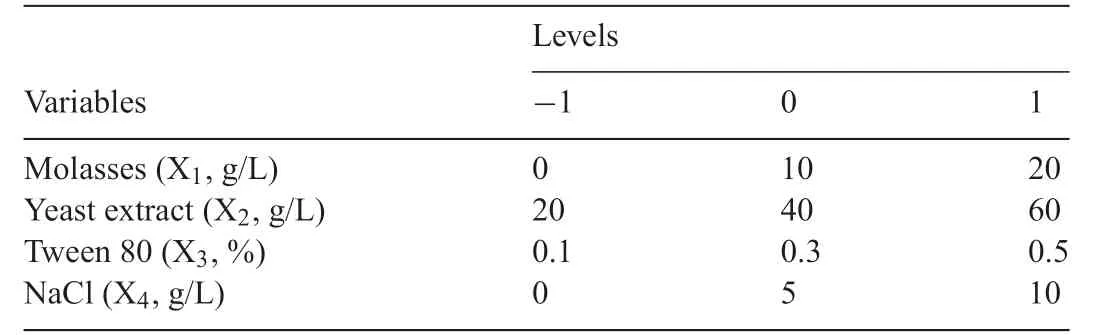
Table1 The response surface methodology experimental range and level of the four independent variables.X1:molasses(0-20,g/L);X2:yeast extract(20-60,g/L);X3:Tween 80(0.1-0.5,%);X4:NaCl(0-10,g/L).
3.5.The optimization of the composition of the media
Previous studies have shown that molasses and Tween 80 have the most significan effect on LycogenTMproduction byR.sphaeroidesWL-APD911,which are the factors for RSM analysis.It has been shown that RSM can be used to optimize the composition of the medium for LycogenTMproduction.Four variables - X1(molasses),X2(yeast extract),X3(Tween 80)and X4(NaCl) - were optimized using RSM to determine the effect of each of these factors.The ranges and the levels of the variables that are used for RSM are listed in Table1.A total of 27 experiments with different combinations of molasses,yeast extract,Tween 80 and NaCl were performed,and the effect of these independent variables on cell growth and LycogenTMproduction is shown in Table2.It is seen that treatment runs 5 and 8 produce a greater integral area of the LycogenTM(≥3100 area/L).ANOVAanalysisshowsthatmolassesandyeastextract have a significan effect on cell growth and LycogenTMproduction.Yeast extract contains a mixture of amino acids,peptides,water-soluble vitamins and carbohydrates.It has been demonstrated that yeast extract promotes cell growth inR.sphaeroides[21].
3.6.The response surface methodology curves for cell growth and LycogenTM production by R.sphaeroides WL-APD911
We presented the cell density and LycogenTMin a designed culture medium as a function of molasses and yeast extract(Fig.5).The RSM data shows that there is maximum cell density and LycogenTMproduction when the concentration of yeast extract is between 40 and 45 and that for molasses is between 10 and 15.It has been shown that the addition of molasses and yeast extract in the culture medium promotes rapid growth and high cell yields for bothP.fluorescensP-5 and P-6[22,23].RSM analysis shows that optimal LycogenTMproduction is achieved at a molasses concentration of 10.0 g/L,a yeast extract concentration of 40.0 g/L,0.3%Tween 80 and a NaCl concentration of 5.0 g/L.
3.7.Large-scale production in a fermenter
Fig.6 shows the batch profil for cell density and LycogenTMproduction inR.sphaeroidesWL-APD911,where production is scaled up from a shake flas process to a 5.0 L and 20.0 L fermenter with an optimal culture.As shown in Fig.6(A),the cell growth enters a stationary phase within 30 h of culture.LycogenTMproduction also increases when cell growth increases.It is worthy of note that the best conditions are achieved at 27 h,which means that a long-term culture could hinder the efficien y.When scaled up to a 20 L fermenter,the cell density increases rapidly within 8 h ofincubation.After cultivation for 34 h,cell growth gradually enters a stationary phase.The maximum cell density is achieved at 34 h after initial cultivation,with a cell density of greater than 10.4 g/L.LycogenTMproduction also follows the same trend as the cell density ofR.sphaeroidesWL-APD911,using the 5 L fermenter system.This phenomenon is also seen in the 20 L fermenter system,with the best density occurring after 30 h of cultivation.The greatest production occurs after 34 h ofincubation.These results show that the scaling up of the fermentation process for cell growth and LycogenTMproduction ofR.sphaeroidesWL-APD911 is successful.
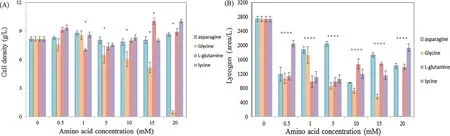
Fig.3.Effects of various nitrogen sources on the cell growth and LycogenTM production of R.sphaeroides WL-APD911.The baterial cell dry weight and LycogenTM productionamountaretestedaccordingtoMaterialsandMethods.(A)celldensity;(B)LycogenTM.Dataarepresentedasmeans±SDofthreeindependentexperiments.*p <0.05.
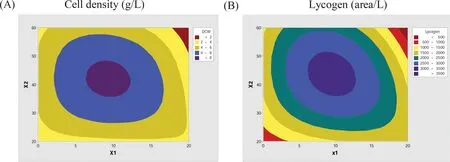
Fig.5.Effects of the response surface methodology experimental design of LycogenTM production by R.sphaeroides WL-APD911 in modifie LB medium as a function of molasses and yeast extract.Data are presented as means±SD of three independent experiments.
3.8.Extraction conditions
Fig.7 shows the effect of the amount of acetone on the LycogenTMextraction fromR.sphaeroidesWL-APD911.The figur shows that the extraction efficien y for LycogenTMincreases rapidly and then becomes almost constant.It has been shown that the type and volume of the extraction solvent usually has a significan effect on the extraction efficien y[24].Organic solvents,including acetone,ethanol,ethyl acetate,chloroform,tetrahydrofuran and methanol,are widely used to extract LycogenTM.However,the polar solvent,acetone,has been shown to be a highly selective reagent for the extraction of carotenoids[25,26].
Food supplements are normally understood include amino acids,fatty acids,fibers minerals,and vitamins,or among other substances to help human health in daily life[27-29].We sup-pose this synthetic biological LycogenTMis beneficia to public health in dietary pattern with a green production strategy.

Fig.6.Bacterial cell growth and LycogenTM production of R.sphaeroides WL-APD911 are scaled up at the optimal culture conditions with(A)5.0 L fermentor;(B)20.0 L fermentor.Data are presented as means±SD of three independent experiments.
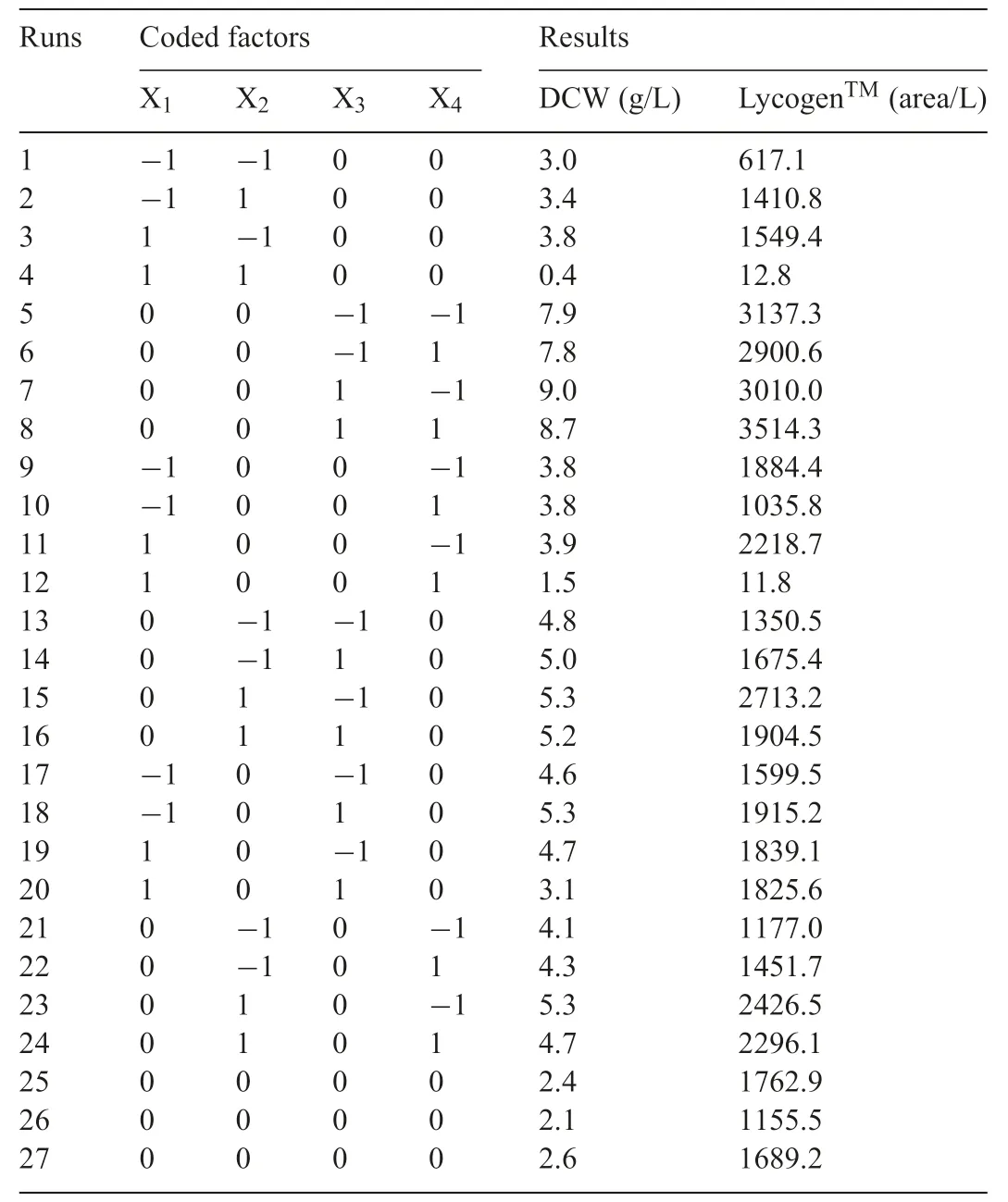
Table2 The response surface methodology experimental design and results for production cell dry density and LycogenTM by R.sphaeroides WL-APD911.Dry cell weight:DCW,g/L.According to the RSM rule,there are 27 independent experiments and results.
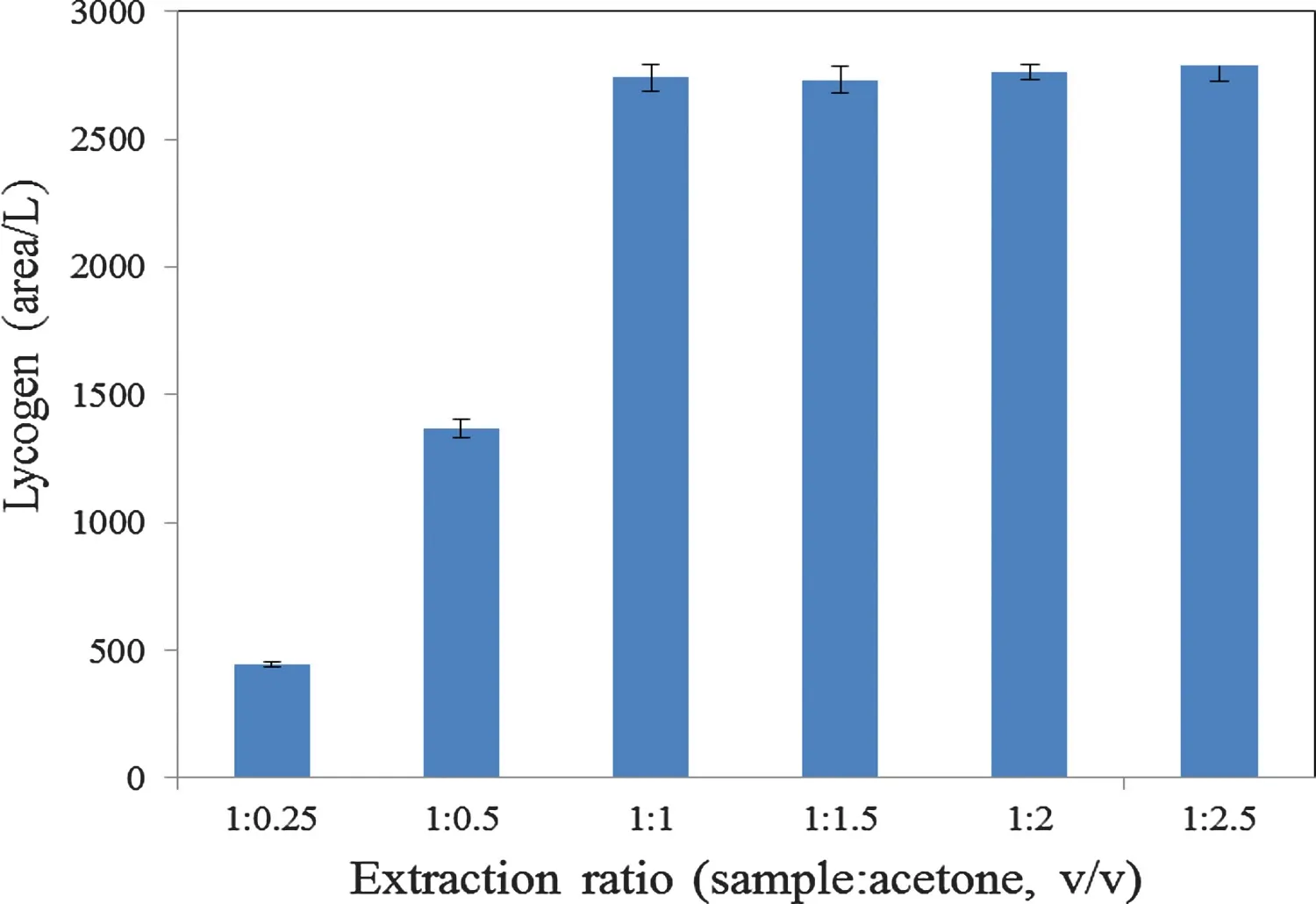
Fig.7.Effects of the extraction ratios on the LycogenTM production of R.sphaeroides WL-APD911.The quality of LycogenTM production is tested according to Materials and Methods.Data are presented as means±SD of three independent experiments.
4.Conclusions
In the present study,the supplementation of LB medium with molasses and Tween 80 increased the production of LycogenTMbyR.sphaeroidesWL-APD911.RSM indicated that maximum LycogenTMproduction was achieved at a molasses concentration of 10.0 g/L,a yeast extract concentration of 40.0 g/L,0.3%Tween 80 and a NaCl concentration of 5.0 g/L.When scale-up to a 20.0 L fermentor,R.sphaeroidesWL-APD911 reached the stationary phase after 34 h of cultivation,coinciding with maximum cell density LycogenTMproduction.These parameters provided an important foundation for the optimization and industrial scale up process of cell growth and LycogenTMproduction.
Conflict ofinterests
The authors declare that there is no financia conflic ofinterests to publish these results.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology,Taiwan,ROC (MOST 104-2221-E-005-096-MY2; and MOST 104-2628-E-005-004-MY3).We thank the projects of Center for Stem Cell Research,Kaohsiung Medical University,Kaohsiung,Taiwan,KMU-TP104G00 and KMU-TP104G02-05.The financia supports were also from KMU-DK105005 and NSYSUKMU105-P 007.
- 食品科学与人类健康(英文)的其它文章
- Effects of Soursop flwers(Annona muricata L.)extract on chemical changes of refine palm olein stored at frying temperature
- About the Beijing Academy of Food Sciences
- Therapeutic molecules for multiple human diseases identifie from pigeon pea(Cajanus cajan L.Millsp.)through GC-MS and molecular docking
- Establishment of probabilistic model for Salmonella Enteritidis growth and inactivation under acid and osmotic pressure
- Terminalia arjuna:A novel natural preservative for improved lipid oxidative stability and storage quality of muscle foods
- Comparison of chicoric acid,and its metabolites caffeic acid and caftaric acid:In vitro protection of biological macromolecules and inflammator responses in BV2 microglial cells

