Effects of Soursop flwers(Annona muricata L.)extract on chemical changes of refine palm olein stored at frying temperature
Tonfak Djikeng Fabrie,Womeni Hilaire Maaire,Bala Krishna Marrapu,Mallampalli Sri Lakshmi Karuna,Rahapui Baari Narayana Prasa,Liner Mihel
a School of Agriculture and Natural Resources,Catholic University Institute of Buea,P.O BOX 563,Buea,Cameroon
b Department of Biochemistry,Faculty of Science,University of Dschang,P.O BOX 67,Dschang,Cameroon
c CSIR-Indian Institute of Chemical Technology,Centre for Lipid Research,Tarnaka,Hyderabad 500007,India
d Biomolecular Engineering Laboratory(LIBio),University of Lorraine,ENSAIA,Vandoeuvre-les-Nancy,France
Abstract In this study,the antioxidant activity of soursop flwers extract in delaying palm olein oxidation at frying temperature was assessed.The oil was supplemented with the extract at concentrations 200-1800 ppm,and stored in the oven at 180°C for 6 days (4 h heating per day).Palm olein containing butylated hydroxytoluene(BHT)served as positive control while the same oil without antioxidant(Control)served as negative one,in order to monitor changes in oils.After each two heating days,oil samples were collected,and their chemical indexes were determined.Peroxide,para-anisidine,TOTOX,thiobarbituric acid and iodine values were the parameters evaluated.Additionally,the evolution of the fatty acid composition of each oil sample during the storage was assessed by gas-chromatography using a flam ionization detector(GC/FID).Generally,palm olein samples containing the natural extract have exhibited the lowest rate of oxidation,and were efficien in limiting the destruction of unsaturated fatty acids in oil at frying temperature.The order of effectiveness in inhibiting oil oxidation was the following:PO+AnM1800ppm>PO+AnM1400ppm>PO+AnM1000ppm >PO+An.M600ppm>PO+An.M200ppm>PO+BHT200ppm=Control.From these results,it can be concluded that soursop flwers are a potent sources ofinatural antioxidants for stabilization of palm olein.
Keywords: Soursop flwers;Stabilization;Natural antioxidant;Palm olein;Frying;Oxidative stability
1.Introduction
Refine Palm olein is intensively used in frying processes[1]for manufacturing fast foods,due to its good oxidative stability[2].The fatty acids of this oil(saturated and unsaturated)were found to contribute positively on the stability of fried foods fla vor [3],compared to other frying oils available in the market[4].Despite its good oxidative stability,high processing temperature can lead to the reduction ofits quality.It is generally accepted that oils easily decompose when heated at frying temperature.During the frying process,physicochemical reactions such as thermooxidation,lipolysis,polymerization,isomerisation or cyclization occur in oil due to elevated temperatures.Consequently,the oil lost its quality in favour to primary and secondary oxidation products,which can affect the organoleptic properties of the fried products[5].Oxidation products of fatty acids lead to off-flvors and odors in the frying bath and fried products [6].They are also dangerous for consumers,because of their implication in many health risks such as,heart diseases,cancers etc[5].
To encounter the reactive oxidation species,chemically synthesized antioxidants are added in adequate amounts in oils and fats with the objective to stop the appearance of off-flvors released during the oxidation of unsaturated fatty acids.Some examples of these additives are:butylated hydroxytoluene,butylated hydroxyanisole andter-butylhydroquinone[1].However,the use of these synthetic additives is strictly controlled,and the increment of consumers awareness for food additives and safety has increased the interest to use natural antioxidants as alternative to synthetic ones [7].Additionally,these synthetic antioxidantshavebeenproventobelessstableathighprocessing temperature[8-11].
In previous studies,it has been reported that natural plant extracts have different degrees of antioxidant properties in fats and oils [1,11-13].In these studies,authors have related the observed activities to the presence in these plants of bioactive components such as phenolic compounds which are known as good antioxidants.Soursop tree(leaves,barks and fruits)is used in natural medicine in the tropics because ofits multiple health benefieffects[14,15].In many studies,the phytochemical composition and antioxidant potential ofleaves,barks and fruits of this plant have been reported[14,16-18].Additionally,Womeni et al.[11]have reported that the methanolic extract of the flwers of this plant is rich in phenolic antioxidants.These authors found that the total phenolic content of soursop flwers was 51.33 mg/g.The antioxidants detected in the same extract by high performance liquid chromatography were vanillic acid,caffeic acid,ferulic acid,ellagic acid and quercetine.Womeni et al.[11]have also proven that this extract is a good radical scavenger and ferric reducer,and,was efficien as antioxidant in stabilizing palm olein during 30 days storage at 70°C.Also,the ability ofinatural plant extracts in limiting oil adulteration during frying or storage at frying temperature have already been demonstrated[12,19].Since palm olein is used for frying in different locations in the world,the knowledge of the potential effects of soursop flwersextractonitsstabilityduringprocessingatfryingtemperature will be of very good interest.So,this work was designed to investigate the effect of different concentrations of soursop flwers in inhibiting palm olein adulteration during storage at frying temperature.
2.Material and methods
2.1.Material
The reagents and chemicals used in this study were of analytical grade.They were procured from HiMedia Laboratories Pvt.Ltd,Sd Fine Chemicals,Mumbai,India and Sigma-Aldrich,St; Louis,USA.The standards fatty acids methyl esters were provided by Sigma-Aldrich,St.Louis,USA.
Refine palm olein without additive (antioxidants) was obtained from SCS/RAFCA Palm Oil Industry Company Ltd,based at Bafoussam,West-Cameroon.The fresh flwers of soursop tree (Annona muricata) were harvested in Dschang,West-Cameroon in March 2013.
2.2.Methods
2.2.1.Extraction of antioxidants from soursop flowers
The fresh flwers were cleaned and dried in an electric airdried oven at 50°C for 48 h.They were then grounded and sieved using a 1 mm diameter sieve.100 g of the powder was macerated at room temperature in 800 ml of methanol with regular shaking for 48 h.After filtratio using the N°1 Wattman paper,the residues were again macerated in 400 ml of methanol,in order to maximize the extraction of phenolic antioxidants.The obtained filtrat was mixed with the previous one,before eliminating the solvent on a rotatory evaporator at 40°C under reduced pressure.The concentrated extract was stored in the refrigerator at 4°C for further analysis.
2.2.2.Samples preparation
The samples were prepared according to the method described by Iqbal et al.[20].The concentrated methanolic extract was dissolved in 1 ml of solvent (methanol) and individually added in 100 g of preheated refine palm olein without additives(antioxidants)(at 50°C for 3 h)at f ve differents concentrations (200,600,1000,1400 and 1800 mg/Kg or ppm).Butylated hydroxytoluene used at its legal concentration(200 mg/Kg or ppm)[21]served as positive control in order to compare the preservative property of the extract.Refine palm olein without additives (antioxidants) and prepared under the same conditions serve as negative control.It is important to note that,the concentration of added methanol (1 mL/100 g of oil)was equal or less than 10 mg/kg(In Europe)and 50 mg/kg(In Japan),which are the recommended amount of methanol to be added per kilogramme of oil and other foods as supplement and additive respectively [22-24].However,after shaking the oils for 30 min,they were stored in the oven at 45°C for 48 h in order to reduce the amount of the added methanol to a value less than 10 mg/kg as recommended by the regulations[22-24].After this storage,oils samples were used for the Schaal oven test.
2.2.3.Schaal oven test
The method described by Sultana et al.[25]with slight modification was used.The prepared oil samples were stored in an electric air-dried oven at 180°C for 6 consecutive days(4 h heating per day).Oil samples were collected after every two days and placed in the refrigerator for further analysis.Their stability towards oxidation was evaluated by measuring the primary and secondary oxidation products.The parameters analyzed are cited below.The changes in the fatty acid profil of each oil sample during storage was also evaluated.
2.2.4.Measurement of oxidation indexes
Peroxide value of each oil sample was determined according to the spectrophotometrical IDF standard method,74A:1991 [26]; The secondary oxidation products were measured using thiobarbituric acid andp-anisidine values,as described by Draper and Hadley[27]and the AOCS officia method guide CD 18-90[28]respectively.The iodine value was also evaluated by the AOCS officia method,but the CD 1-25(AOCS,2003).The total oxidation(TOTOX)of different treatments was calculated from their peroxide andp-anisidine values using the following equation:TOTOX=2PV+AV[29].
2.2.5.Effect of the extract on the fatty acid profile of oil during the storage 2.2.5.1.Fatty acid methyl esters preparation.Fatty acid methyl esters (FAMEs) of stabilized and control palm olein samples were prepared by transesterificatio using 2%sulphuric acid in methanol [30].The FAMEs were extracted into ethyl acetate and thoroughly washed with water to make them free of acid,and dried over anhydrous sodium sulphate.The dried esters were analyzed in a gas-chromatograph using a flam ionization detector(GC/FID).
2.2.5.2.Gas chromatography.The analysis of the fatty acid methyl esters was made on an Agilent gas chromatograph(Agilent Technologies,Palo Alto,CA,USA,N°of serie 7890A),usingaflam ionizationdetector,andaDB-225capillarycolumn(30 m x 0.25 μm of fil thickness).Initially,the temperature of the column was maintained at 160°C for 2 min.After,it increases to 220°C (5°C/min) and was finall maintained at 220°C for 10 min.The mobile phase was nitrogen,and its flw rate was 1.5 mL/min.The temperature of the detector and injector were respectively 250 and 230°C.The fatty acids were identifie by comparing their retention times to that of standards fatty acids methyl esters,analyzed under the same conditions.
2.2.6.Statistical analysis
Results obtained in this study were subjected to one-way analysis of variance (ANOVA) with Dunnet and Student-Newman-Keuls tests using Graphpad-InStat version 3.05,to evaluate the statistical significanc of the data.The differences were significan at probabiliy level less than 5%.
3.Results and discussion
3.1.Peroxide value
Increase in peroxide value(PV)generally reveals the formation of hydroperoxides during oil oxidation.Peroxide value is measured by researchers working on antioxidants to evaluate the stageofprimaryoxidationinlipids[11,13,19,31-33].The trends in peroxide value of palm olein samples supplemented with antioxidants in comparison with control are given in Table1.A significan increase(p<0.05)in PV was registered in all the samples during the firs four days of storage.From the fourth to the sixth day of heating,the PV of oils samples containing antioxidants was still increasing while that of the control started decreasing.The PV of the sample PO+BHT200ppmwas significantly lower(p<0.05)than those of oils enriched with natural antioxidants.The increase in peroxide value registered in all the samples during the storage indicates the formation of hydroperoxides.It has been proven that at high processing temperature,oxidation reactions are easily initiated because,the hydrogenswith weakest bonds on the carbon of unsaturated fatty acids are removed,leadingtofreeradicals(alkylradicals),whichcanreact with molecular oxygen to form peroxyl radicals.These radicals are relatively unstable,and can remove the allylic hydrogen from another fatty acid to form hydroperoxides[34].The significan decrease in PV observed in the control sample might be the sign ofits higher alteration compared to the other oil samples,due to the breakdown of hydroperoxides into secondary products[35]or into other radicals like alkoxy and hydroxy [34].The relative increase in PV of stabilized palm olein samples could be attributed to their low secondary oxidation,due to the presence of antioxidants.It should be noted that the peroxide value is not a proper indicator for evaluation of oil quality changes during frying,due to the rapid decomposition of hydroperoxides at high temperature.However,it can be an indicator of oil instability if the secondary oxidation products are also measured.This will help to check if these peroxides are decomposed in favour to secondary oxidation products.We cannot directly conclude from these results on the good stability of oil supplemented with antioxidants,because low peroxide value can be related to good or bad oil quality.Furthermore,high processing temperature can also significantl influenc the antioxidant,causing a loss in its activity due to thermal decomposition[8].However,this plant extract has already been proven in our previous work that it is stable at high processing temperatures and can serve as source ofinatural antioxidants for delaying peroxide formation in palm olein during 30 days storage at 70°C[11].These results are in line with those of Che Man and Tan [19],who noticed similar effects in palm olein supplemented with natural extracts of oleoresin rosemary and sage during frying of potatoes chips.Raza et al.[12]showed that,methanol extracts ofAlthea rosea,Chemopodium album,Fumaria indicaandCichorium intybuswere efficien in limiting peroxide formation in sunflwer oil during storage for 60 min at frying temperature.
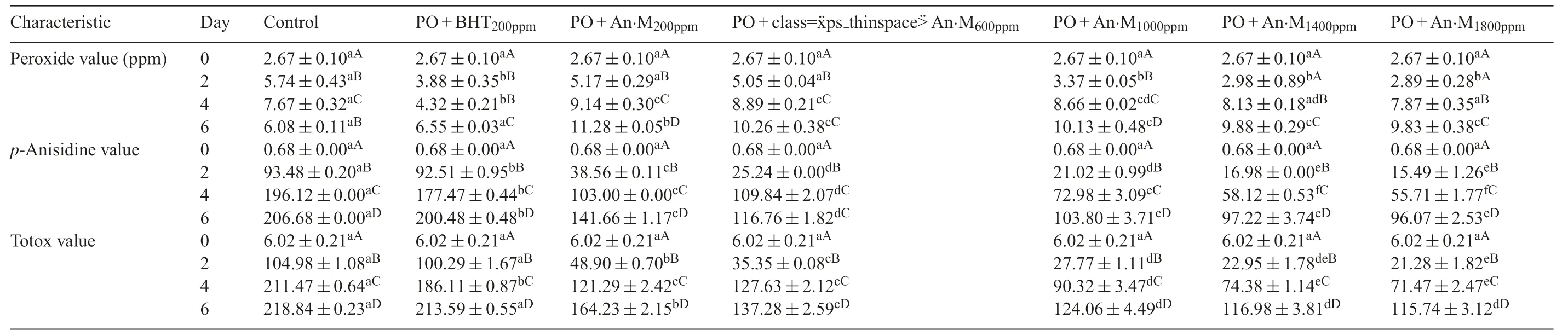
Table1 Changes in peroxide,p-anisidine and TOTOX values of RBD palm olein during 6 days storage at 180°C.
3.2.p-anisidine value
During oxidation oflipids,products formed at the primary stage can be converted,depending on the processing or storage temperature,into secondary oxidation products,especially 2-alkenals and 2,4-dienals,which can be measured using anisidine test[1].The secondary oxidation state of stabilized and control palm olein samples stored at frying temperature for 6 days is presented in Table1.A relative increase inp-anisidine value was registered in all the samples.Control and PO+BHT200ppmexhibited the highest secondary oxidation states during storage.The oxidation state of these two samples was not statistically different(p>0.05),but higher(p<0.001)than that of palm olein samples enriched with soursop extract as preservative.The highestp-anisidine value in control and PO+BHT200ppmindicates highalteration.Thelackofantioxidantinthecontrolsample,and the thermal instability of BHT might explain the observed alterations[8,10].Oil samples containing different concentrations of soursop flwers were relatively resistant to secondary oxidation.Antioxidant compounds of this extract might be responsible for this resistance.In fact,Womeni et al.[11]have demonstrated that the total phenolic content of soursop flwers methanolic extractis 51.33 mg GAE/g.They have also detected the presence of vanillic acid,caffeic acid,ferulic acid,ellagic acid and quercetine in this extract.These are phenolic antioxidants,famous for their good antioxidant activities.It has also been shown that this extract is a powerful radical scavenger due to its ability of the antioxidants present to donate their hydrogen atom.They might stabilize free radical formed in palm olein through similar mechanism of action.Hence,this extract was the best in preventing secondary oxidation in palm olein than BHT.The fact that BHT could be less stable at high processing temperatures than the extract has also been already proven by Womeni et al.[11]using Rancimat test.This is also supported by the findin Chang et al.[8]and Thorat et al.[10]who showed BHT and BHA are not stables at high processing temperature.These results are also supported by those of Iqbal and Bhanger [36]and Iqbal et al.[20]which showed that methanol extract of garlic and pomegranate respectively are more stable at 185°C than BHA.The results ofp-anisidine confir the fact that peroxide value only,cannot help to confir the oxidative status of oil at frying temperature,because of the rapid conversion the peroxides formed into secondary oxidation products.The lower peroxide value of PO+BHT200ppmwas then the consequence of the high decomposition of hydroperoxides.Results of these investigations show that the extract ofAnnona muricatacan reduce primary and secondary oxidation formation in palm olein sored at frying temperature.Similar results were obtained with different plant extracts in the same or different oil systems[12,19].

Table2 Changes in TBA,iodine values and linoleic acid content of RBD palm olein during 6 days storage at 180°C.
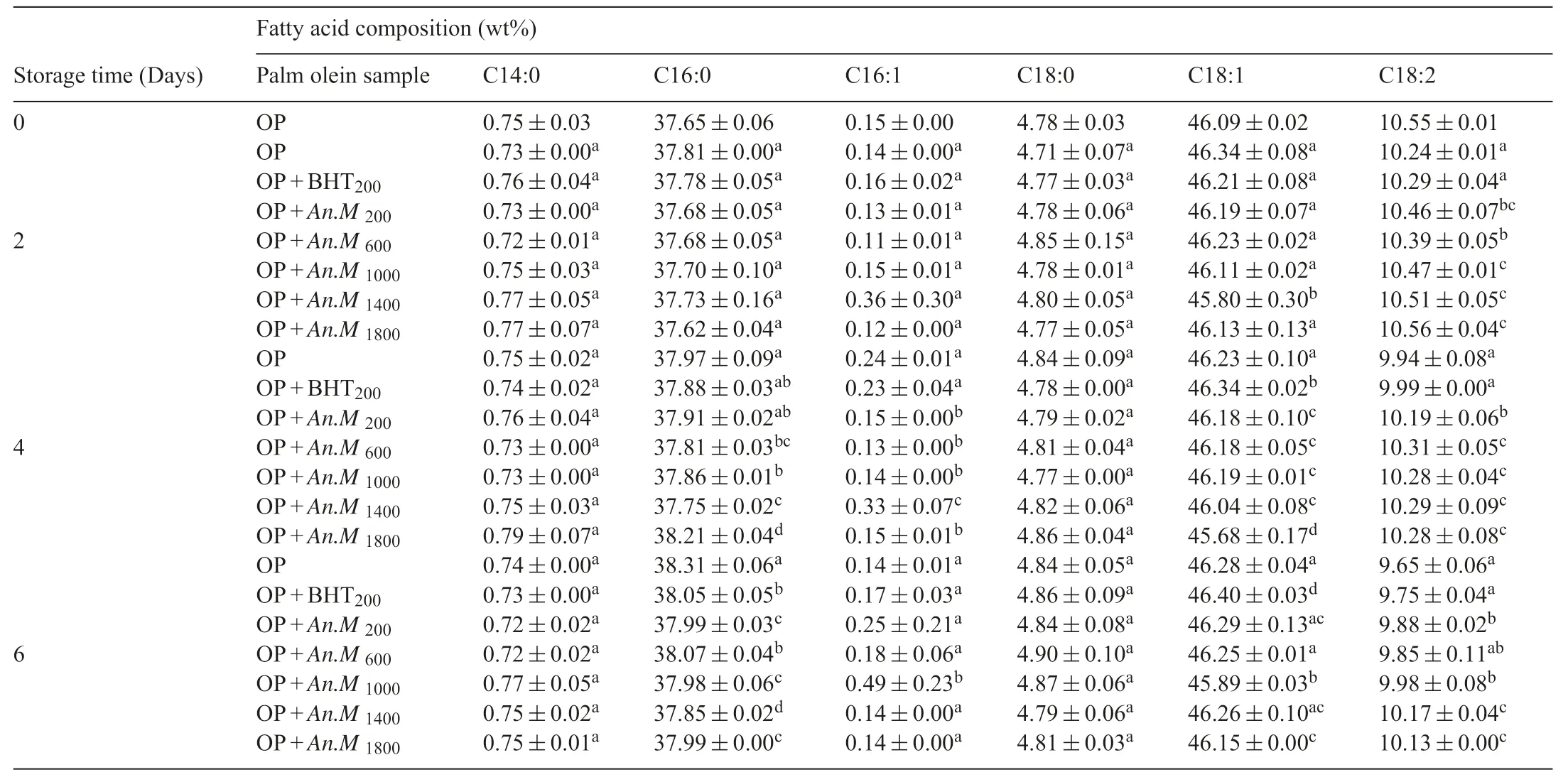
Table3 Fatty acid profil of palm olein supplemented with soursop flwers extract(Annona muricata)during the storage at frying temperature.
3.3.TOTOX value
Total oxidation (TOTOX) of palm olein samples supplemented with antioxidants in comparison with control is shown in Table1.Generally,a significan increase in total oxidation was registered in all the samples during the experiment.Palm olein(control)and PO+BHT200ppmwere more oxidized than oil samples stabilized with natural antioxidants.This is because their TOTOX values were higher than those of oil samples containing the extract.As previously mentioned,the lack of antioxidants in control might explain its high oxidation state,while that of PO+BHT200ppmcould be due to its thermal instability at high temperature as proven by Chang et al.[8]and Thorat et al.[10].The lower oxidation state of palm olein containing natural antioxidants might be the consequence of the action of antioxidants compounds presents in this extract,as reported by Womeni et al.[11].It is also seen in Table1 that the effect of the extract was increasing with the concentration;because at high concentration of extract(1000-1800 ppm),the total oxidation was very low.Similar results were observed by Iqbal and Bhanger [36]and Iqbal et al.[20]who have respectively demonstrated that the protective effect of garlic and pomegranate extract towards the oxidation of sunflwer oil was increasing with their concentration.Results of this study,showing that natural plants extracts can significantl reduce the alteration of oils at frying temperature are in accordance with those reported in the litterature[19,32].
3.4.Thiobarbituric acid value
Thiobarbituric acid value is the condensation reaction between thiobarbituric acid and malonaldehyde,which are the most predominant product of the secondary oxidation of fatty acids in food lipids.Therefore,it is considered as a good chemical criterion to check the oxidative state of fresh oils and fats[37].Variations in TBA values of palm olein samples are presented in Table2.The highest TBA values were depicted in the Control and PO+BHT200ppmwhile those of palm olein samples containing plant extract as preservative were relatively stable.This suggests that,the accumulation of malondialdehyde in palm olein samples stabilized with the natural extract was lower than those of control and PO+BHT200ppm.This indicates the efficien y of the extract in limiting secondary oxidation in palm olein at frying temperature.This is also the proof that,soursop flwers antioxidants have good stability at high temperature.As previously mentioned,the good stability of oil samples supplemented with the extracts compared to control and OP+BHT200ppmmight be attributed to the phenolic antioxidants present in this extract [11].For palm olein sample supplemented with BHT,the thermal instability of this compound might be responsible of the rapid accumulation of malondialdehyde,while,the lack of antioxidant might explain this accumulation in control.This result is in accordance with the findin of Che Man and Tan[19],who demonstrated that the extract of oleoresin rosemary and sage were more efficien than BHT and BHA in delaying malondialdehyde formation in palm olein during frying of potatoes chips.
3.5.Iodine value
This parameter is generally used to have an idea of the degree of unsaturation of oils and fats.Its decrement is consistent with the destruction of unsaturated fatty acids,when oil becomes oxidized[38].Changes in iodine value of different oil samples during the storage at frying temperature are presented in Table2.The iodine value decreased in almost all the samples during storage.The highest rate of decrement was registered in control and PO+BHT200ppmwhile that of oil samples containingAnnona muricataextract was the lowest.A significan change in the iodine value of the control and PO+BHT200ppmcompared to oil containing the extract as antioxidants indicates that the oxidation rate of unsaturated fatty acids is elevated,probably due to the absence of antioxidants in the control and to the thermal instability of BHT which might have completely lost its activity at this processing temperature.Consequently,oil becomes unprotected and abandoned for free radical actions.The lowest changes in iodine value in oil containing natural antioxidants compared to control and PO+BHT200ppmis an indication of the low destruction of their unsaturated fatty acids,due to the ability of antioxidant molecules presents to limit the deteriorative effect of free radicals,by donating hydrogen atoms for their stabilization.Therefore,the changes in iodine value makes soursop extract to be most effective in protecting unsaturated fatty acids of palm olein from oxidation compared to BHT.The same observations were made in palm olein by Che man and Tan[19]with the extract of oleoresin and sage during frying of potato chips.However,these results were contradictory to those found by Raza et al.[12]in sunflwer oil using the methanolic extract ofChemopodium album,Althea rosea,Fumaria indicaandCichorium intybusduring an accelerated storage of 60 min at frying temperature.These authors demonstrated that BHT was more efficien in delaying unsaturated fatty acids destruction than natural plant extracts.Processing time and conditions might be responsible of these differences.
3.6.Effects of soursop extract on the fatty acid profile of palm olein
The action ofAnnona muricataextract on the fatty acid composition of palm olein samples is given in Table3.No significan variations were found in the amount of myristic(C14:0),palmitic acid (C16:0),palmitoleic acid (C16:1),stearic acid(C18:0) and oleic acid (C18:1) acids profil during the storage time at 180°C.However,a significan decrease (p<0.05)in linoleic acid percent was registered(summarized in Table2).Initially,the linoleic acid amount in unheated palm olein was 10.55% (Table2).This initial percentage decreased gradually with storage,and the higher decrement rate was depicted in control and PO+BHT200ppm.This decrement was less in oil samples containing the natural extract.At the sixth day,the percentages in linoleic acid were 9.65%,9.75%,9.88%,9.85%,9.98%,10.17% and 10.13% respectively in PO (control),PO+BHT200ppm,PO+An·M200ppm,PO+An·M600ppm,PO+An.M1000ppm,PO+An.M1400ppmand PO+An.M1800ppm(Table2).It is clear that,the effect of the extract in protecting linoleic acid from oxidative degradation was concentration dependent.The decrement in linoleic acid percent observed during the storage could be attributed to its oxidation.From Table2,It can be observed that the linoleic acid percent in oil samples containing the natural extracts was higher than that of PO+BHT200ppmand control at the 6th day,demonstrating that linoleic acid alteration was retarded by the extracts,at all concentrations.These outputs are in accordance with those reported by Che man and Tan [19],which registered a similar decrease in linoleic acid of palm olein containing oleoresin rosemary and sage extracts as natural antioxidants.Their results also showed that these natural extracts were most efficien in retarding linoleic acid oxidation(decrease in its percentage)than BHA at the last frying day.The effica y and good thermal stability ofannona muricataantioxidants might explain its good protective effect,while,the lack of antioxidant and instability of BHT at high temperature respectively can explain their loss in linoleic acid.However,the loss oflinoleic acid observed in this study was most important than that obtained in our previous work with the same oil and extract[11].The difference in processing temperature might explain these variations.
4.Conclusion
From these results,it is reasonable to say that methanolic extract of soursop flwers is a potent source of antioxidants which can be used to improve the stability of palm olein during storage at frying temperature.This extract was efficien in delaying primary and secondary oxidation products formation in palm olein,than BHT.It was also most efficien in limiting unsaturated fatty acids destruction during the treatment.Its activity increases with the concentration.Hence,the agro material tested can be a viable source ofinatural antioxidants for stabilization of oils and fats.However,it is recommended to investigate on the toxicity of this extract before their application in vegetable oils and functional foods.
Formatting of funding sources
This research did not receive any specifigrant from funding agencies in the public,commercial,or not-for-profisectors.
Conflict ofinterest
None
Acknowledgment
The authors would like to thank the Ministry of External Affairs and the Department of Science and Technology,Government of India,who,through the Federation of Indian Chambers of Commerce finance the realization of parts of this work in India,under the CV Raman International Fellowship for African Researchers in India 2013.
Special thanks go to the World Academy of Science and the Council for Scientifiand Industrial Research,Government of India for facilitating the completion of this project through the TWAS-CSIR Sandwich Fellowship for Postgraduate Research 2014.
- 食品科学与人类健康(英文)的其它文章
- About the Beijing Academy of Food Sciences
- Therapeutic molecules for multiple human diseases identifie from pigeon pea(Cajanus cajan L.Millsp.)through GC-MS and molecular docking
- A synthetic biological secondary metabolite,LycogenTM,produced and extracted from Rhodobacter sphaeroides WL-APD911 in an optimizatioal scale-up strategy
- Establishment of probabilistic model for Salmonella Enteritidis growth and inactivation under acid and osmotic pressure
- Terminalia arjuna:A novel natural preservative for improved lipid oxidative stability and storage quality of muscle foods
- Comparison of chicoric acid,and its metabolites caffeic acid and caftaric acid:In vitro protection of biological macromolecules and inflammator responses in BV2 microglial cells

