芳香烃受体在心房颤动患者心房组织中的表达及意义*
雷建明, 肖 骅, 李 强, 魏 潇, 郭静文, 肖铭汉
(重庆医科大学附属第一医院心血管内科, 重庆 400016)
芳香烃受体在心房颤动患者心房组织中的表达及意义*
雷建明, 肖 骅△, 李 强, 魏 潇, 郭静文, 肖铭汉
(重庆医科大学附属第一医院心血管内科, 重庆 400016)
目的: 检测芳香烃受体(AhR)在风湿性心脏病(风心病)心房颤动患者右心耳组织中的表达,探讨其在心房纤维化中的作用及意义。方法: 取风心病换瓣手术患者的右心耳组织为实验组,其中风心病窦性心律组25例和风心病慢性房颤组11例;取先天性心脏病(先心病)心脏手术患者的右心耳组织12例作为对照组。采用Masson染色法检测右心耳组织胶原含量,采用免疫组化技术检测AhR、AhR核转位蛋白(ARNT)和CYP1A1蛋白的表达和分布,采用实时荧光定量PCR检测AhR、ARNT和CYP1A1的mRNA表达,采用Western blot检测AhR、ARNT和CYP1A1的蛋白表达。 结果: 与先心病组相比, 风心病窦律组和风心病慢性房颤组胶原含量和AhR、ARNT、CYP1A1的表达明显增高;与风心病窦律组相比,风心病慢性房颤组胶原含量和AhR、ARNT、CYP1A1的表达明显增高(P<0.05)。结论: 风心病患者心房组织中AhR 的表达与纤维化程度相关; AhR/ARNT/CYP1A1在风心病患者中表达增加,可能参与风心病心房纤维化的发生发展。
芳香烃受体; 心房颤动; 纤维化
心房颤动(atrial fibrillation,AF)简称房颤,是临床上最常见的心率失常之一,发病率和死亡率随年龄逐渐增加,尤其合并卒中和心衰等并发症时更高[1]。引起房颤最常见的原因是风湿性心脏病(rheumatic heart disease, RHD),该病极易引起瓣膜病变,进而引发房颤[2]。房颤的发生过程中存在结构重构,心房纤维化是结构重构最突出的特征,然而心房纤维化的具体机制还不明确[3]。
芳香烃受体(aryl hydrocarbon receptor,AhR)是一种配体激活性转录蛋白,激活后与AhR核转位蛋白(AhR nuclear translocator,ARNT)结合,可调节细胞色素酶P450家族成员之一CYP1A1的表达[4-5]。有研究表明激活AhR可诱导小鼠肝纤维化[6]。AhR在人体直肠等部位有表达且发挥作用[7],但在心房中是否表达发挥作用却未见报道。为此,我们收集先心病与风心病患者在体外循环手术中收集右心耳标本,检测组织胶原含量与AhR的表达,探讨AhR在心房纤维化中作用,为房颤的相关防治提供新的方向。
材 料 和 方 法
1 材料
1.1 研究对象 在这项研究中总共选入了48位患者,男17例,女31例,平均年龄为(42.77±14.24)岁。这些患者都是在2015.04~2016.03期间在重庆医科大学附属第一医院进行体外循环手术,排除合并有冠心病、高血压以及糖尿病的患者。将患者分成3组:先天性心脏病(congenital heart disease, CHD)窦性心律(CHD+sinus rhythm)组12例;RHD窦性心律(RHD+sinus rhythm)组25例;RHD持续性房颤(RHD+cAF)组11例。术前通过超声心动图检测左室前后径、左室舒张末径、右房横径和射血分数。在进行研究之前,均已通过患者或患者家属签字同意。所有知情同意书以及协议都通过了本院伦理委员会批准。
1.2 右心耳组织取样 在体外循环手术进行时,于手术室外即时接收体外循环手术中剪除的右心耳组织 约100 mg。将右心耳组织用冰盒运送至实验室,以生理盐水清洗,锡箔纸包裹,放入冻存管于-80 ℃保存。
1.3 主要试剂 抗 AhR和CYP1A1抗体(Abcam);抗ARNT抗体(CST);抗β-actin抗体(Proteintech);总RNA提取试剂盒、RNA逆转录试剂盒、实时荧光定量PCR试剂盒以及AhR、ARNT、CYP1A1和β-actin的引物(TaKaRa);Masson染色试剂盒(南京建成科技有限公司);免疫组化试剂盒(北京中杉金桥生物技术有限公司)。
2 主要方法
2.1 Masson染色 使用Masson染色试剂盒进行Masson染色。石蜡切片脱蜡,入95%、70%、30%乙醇各2 min,蒸馏水2 min。40 ℃水中漂洗2次,各30 s。R1核染液染色60 s,冲洗液冲洗30 s。R2浆染液染色60 s,冲洗液冲洗30 s。R3黄色让叶分色8 min,弃去分色液。R4蓝色复染液染色5 min,无水乙醇冲洗干净。中性树脂封固,镜下观察拍照。每张切片在显微镜下随机选取5个高倍视野(×200)。用Image-Pro Plus 6.0软件,测定胶原容积分数(collage volume fraction,CVF)=(胶原面积/所测视野面积),取平均值。
2.2 免疫组化 采用免疫组化方法检测组织中蛋白表达情况。组织固定后,脱水切片,烘烤,泡缸,用SP9001处理进行组化操作,孵 I 抗AhR(1∶100)、ARNT(1∶100)和CYP1A1(1∶50)。4 ℃过夜,孵 II 抗,DAB显色,苏木精染色,晾干,封片,显微镜下观察拍照。每张切片在显微镜下随机选取5个高倍视野(×400)。
2.3 Western blot 组织蛋白称重记录后放入匀浆管,在冰上剪碎组织。RIPA+PMSF(100∶1)裂解组织30 min,4 ℃离心5 min,收取上清液,BCA法测定样品蛋白浓度。SDS-PAGE凝胶电泳分离、电转至PVDF膜(Millipore),5%脱脂奶粉室温封闭1 h,I 抗(AhR 1∶2 000,ARNT 1∶1 000,CYP1A1 1∶100,β-actin 1∶1 000)4 ℃孵育过夜,IgG II 抗(1∶7 500)室温孵育1 h,ECL法显色。以β-actin作为内参照。电泳条带灰度值使用实验室仪器自带软件Image Lab (Bio-Rad)进行分析,以目的条带灰度值与β-actin条带灰度值之比表示目的蛋白的相对表达量。
2.4 实时荧光定量PCR 将右心房组织在冰上匀浆裂解,用总RNA提取试剂根据说明书提取总RNA。用逆转录试剂盒将RNA逆转录。在实验室机器(Lab7500)上进行10 μL体系扩增。引物序列见表1。以β-actin为内参照。反应体系10 μL,PCR扩增条件为:95 ℃ 30 s; 95 ℃ 5 s,60 ℃(AhR、ARNT和CYP1A1)/62 ℃(ANP)退火30 s,40个循环; 65 ℃缓慢升高至95 ℃,每5 s增加0.5 ℃,分析熔解曲线。扩增得到的Ct值使用2-ΔΔCt法计算目的mRNA的相对表达量。
3 统计学处理
使用SPSS 20.0统计软件进行数据分析。计量资料用均值±标准差(mean±SD)表示。多组间比较采用单因素方差分析(one-way ANOVA)法。以P<0.05为差异有统计学意义。
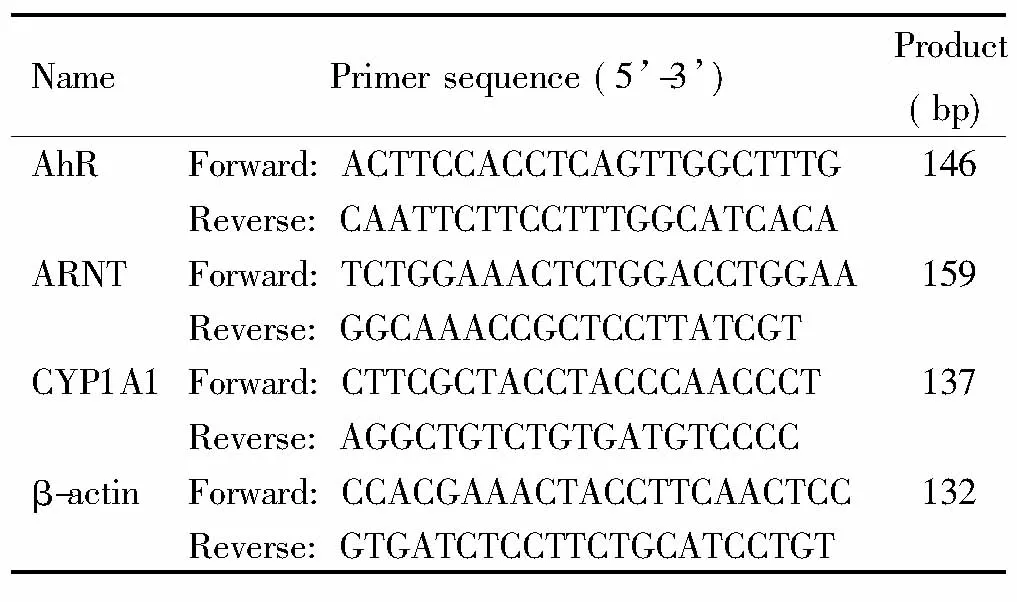
表1 引物序列
结 果
1 患者分组及一般情况
与先心病窦律组相比,风心病窦律及房颤组年龄、左房前后径、左室舒张末径明显增加,右房横径明显减小;与风心病窦律组相比,风心病持续性房颤组左房前后径明显增加,右房横径明显增大(P<0.05)。射血分数在3组间并无显著差异,见表2。
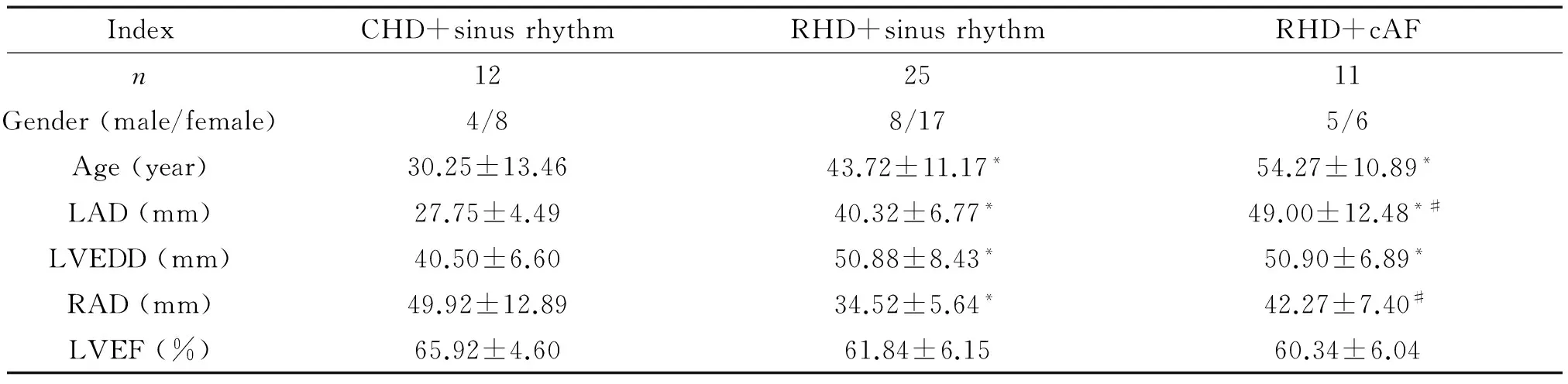
表2 患者一般情况及超声心动图结果
LAD: left atrial dimension; LVEDD: left ventricular end-diastolic dimension; RAD: right atrial dimension; LVEF: left ventricular ejection fraction.*P<0.05vsCHD+sinus rhythm;#P<0.05vsRHD+sinus rhythm.
2 组织胶原容积分数
风心病持续性房颤组心房组织CVF比风心病窦律患者组及先心病窦律组明显增高,风心病窦律组CVF值比先心病窦律患者组明显增高(P<0.05),见图1。
3 AhR/ARNT/CYP1A1在组织中的分布
AhR/ARNT/CYP1A1蛋白在3组患者组织中均有表达,3种蛋白主要分布于胞浆。与先心病窦律组相比,风心病窦律组及持续性房颤组表达增加,其中持续性房颤组增加更为明显,见图2。
4 AhR/ARNT/CYP1A1的蛋白表达
与先心病窦性心律组相比,AhR、ARNT和CYP1A1表达水平在风心病窦律组及风心病持续性房颤组明显升高;与风心病窦律组相比,风心病持续性房颤组明显升高(P<0.05),见图3。
5 AhR/ARNT/CYP1A1 的mRNA相对表达
AhR/ARNT/CYP1A1在风心病房颤组与风心病窦律组中的mRNA表达都显著高于先心病窦律组,风心病持续性房颤组AhR/ARNT/CYP1A1的mRNA表达水平都明显高于风心病窦律组(P<0.05),见图4。
6 相关性分析
AhR在风心病患者中的蛋白表达水平与CVF呈正相关(r=0.67,P<0.05);AhR蛋白表达与ARNT蛋白表达呈正相关(r=0.941,P<0.01);AhR与CYP1A1蛋白表达呈正相关(r=0.939,P<0.01)。
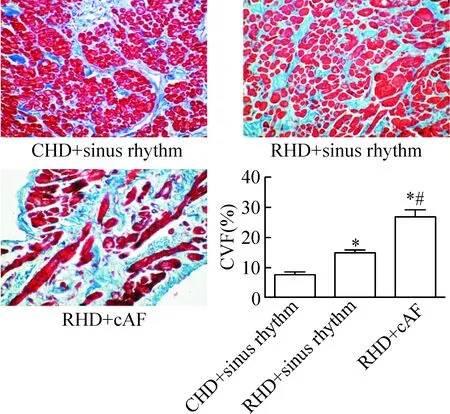
Figure 1.Determination of collagen volume fraction (CVF) in different groups by Masson’s trichrome staining (×200). Mean±SD.n=5.*P<0.05vsCHD+sinus rhythm;#P<0.05vsRHD+sinus rhythm.
图1 胶原染色结果
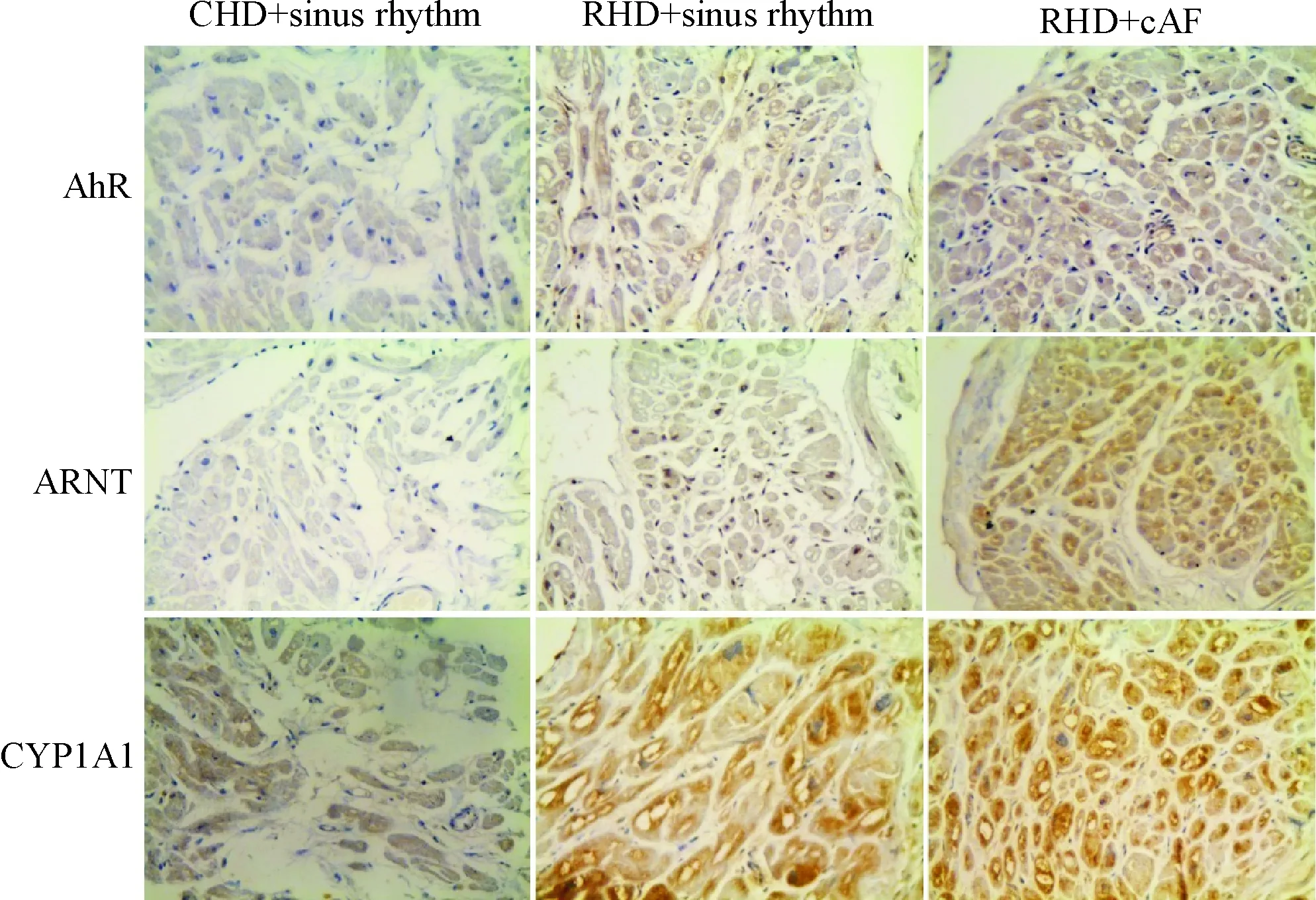
Figure 2.Immunohistochemical analysis of AhR, ARNT and CYP1A1 in CHD+sinus rhythm group, RHD+sinus rhythm group and RHD+cAF group (×400). Brown or yellow stained proteins mainly distributed throughout the cytoplasm of myocardial cells, and their expression increased gradually.
图2 免疫组化结果
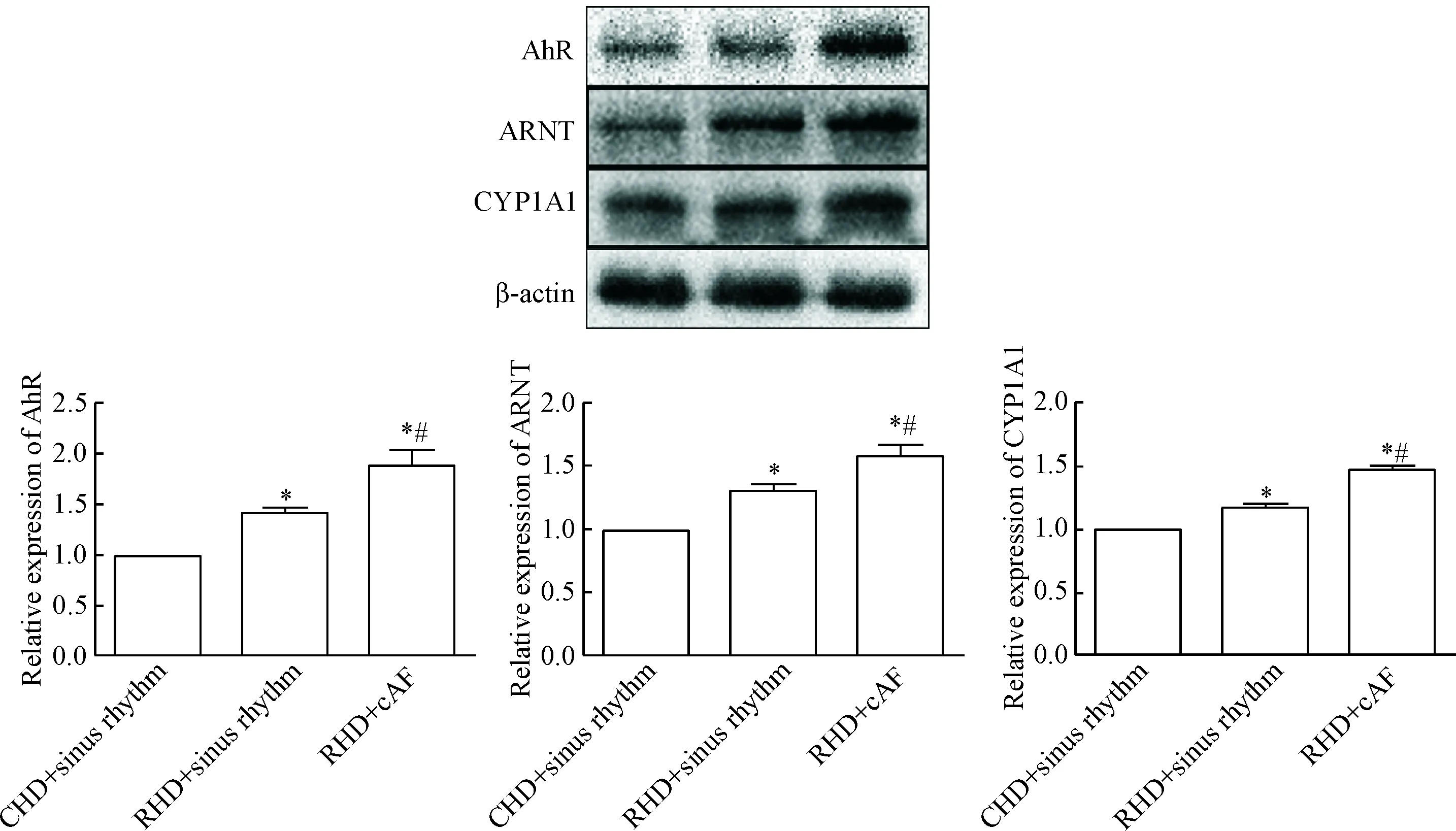
Figure 3.Comparison of the relative protein expression of AhR, ARNT and CYP1A1 among groups. Mean±SD.n=3.*P<0.05vsCHD+sinus rhythm;#P<0.05vsRHD+sinus rhythm.
图3 蛋白表达结果
讨 论
近年来,房颤的发病率和死亡率逐渐升高,造成了巨大的社会负担[8]。继发于风湿性心脏病的房颤很常见,它能够损害心功能、增加体循环栓塞的风险[2]。在将来房颤会有更高的发病率[9]。
心房纤维化是房颤最显著的结构改变[3]。在本研究中心房组织纤维化程度在先心病窦律组、风心病窦律组、房颤组逐渐升高,左房舒张末径也相应增大,同样说明心房纤维化与房颤相关。此发现与Zhang等[10]、Li等[11]的实验结果符合。何文聪等[12]在研究纤维化与缝隙连接重构关系时也有类似发现。风心病房颤组右房横径比风心窦律组明显增高,说明房颤加深心房病变,而先心病组右房横径更高,原因可能是大部分入选先心病人为房间隔缺损,且年龄较大。这些病人,右房比风心病组更大,心率却是窦律,心房纤维化程度较低,这进一步说明房颤与纤维化密切相关。
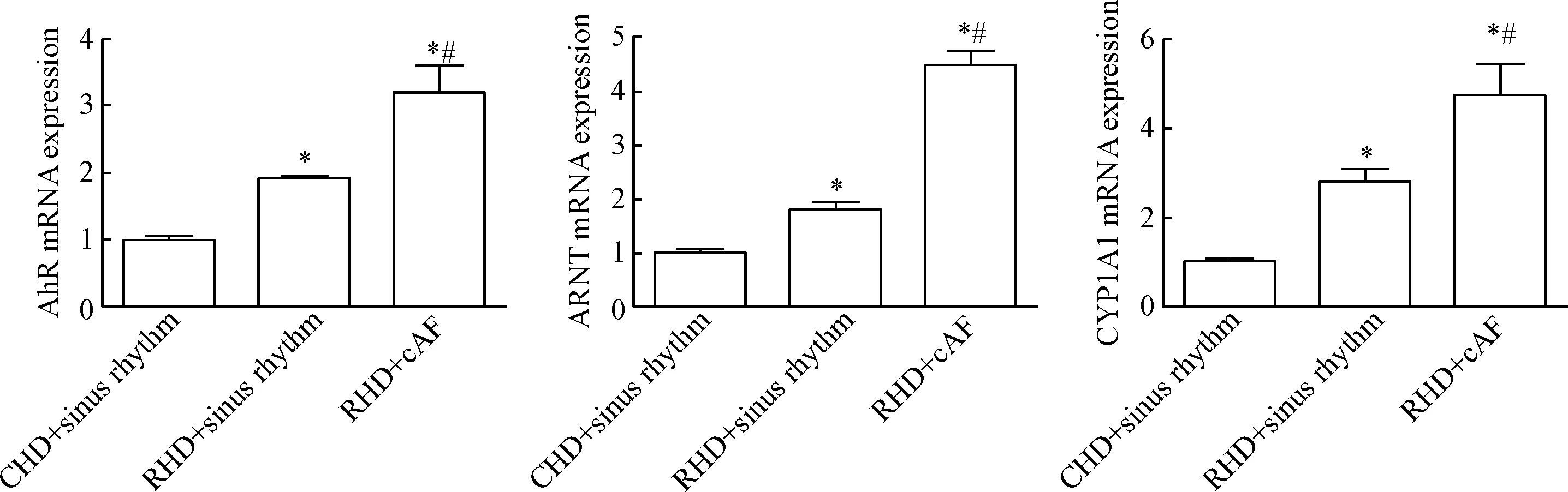
Figure 4.Comparison of the relative mRNA expression of AhR, ARNT and CYP1A1 among groups. Mean±SD.n=5.*P<0.05vsCHD+sinus rhythm;#P<0.05vsRHD+sinus rhythm.
图4 各组mRNA比值
近期研究发现 AhR参与细胞分化、组织重构、免疫调节等过程[13]。AhR/ARNT/CYP1A1是一经典通路,参与纤维化等多种调节。Poormasjedi-Meibod等[14]研究表明在真皮成纤维细胞中AhR/ARNT/CYP1A1参与纤维化。Antos等[15]研究发现TCDD刺激AhR/ARNT/CYP1A1在卵巢中表达增加,发现了通过CYP1A1作为靶点解毒的可能性。涉及AhR的基础研究大部分都是以动物作为模型[16-17],本研究首次在人体心脏组织中发现AhR的表达,且发现在风心病患者中表达显著高于先心病患者,提示AhR可能也是心脏疾病的一个重要因素。在本研究中AhR的表达在先心病窦律组、风心病窦律组、风心病慢性房颤组中逐渐升高,与心房纤维化程度一致。这也与Pierre等[6]在TCDD刺激小鼠肝脏细胞纤维化后,AhR蛋白及mRNA表达增加的结果相符。Xue等[17]也在研究中发现AhR参与调节小鼠胰腺纤维化。这些研究结果提示AhR可能参与心房纤维化的发生发展。ARNT是AhR核转运蛋白,是AhR相关信号通路的一个重要部分,许多基因调控都是通过AhR/ARNT介导的[18-20]。本研究中在3个组ARNT蛋白表达趋势与AhR趋势相同,呈正相关。提示AhR可能是通过介导ARNT参与心房纤维化。CYP1A1是细胞色素酶P450家族成员之一,在化学物质代谢中起着重要作用,也是AhR/ARNT的一个下游调控基因[19]。Yaming等[21]研究发现CYP1A1基因在口腔黏膜下层纤维化中起重要作用,Ghosh等[22]也发现CYP1A1增加口腔黏膜下层纤维化的风险。本研究中,纤维化组织中CYP1A1蛋白表达趋势与AhR/ARNT蛋白表达趋势相一致,呈正相关。提示CYP1A1可能是AhR下游调控心房纤维化的关键环节,可能成为治疗风心病房颤患者防治心房纤维化的重要靶点。
综上所述,AhR/ARNT/CYP1A1信号通路可能参与了风心病房颤患者心房纤维化的发生发展。本研究证实了AhR在人体心房组织中的表达,并发现AhR的表达与组织纤维化程度相关,扩充了在人体心脏领域中对AhR的研究,丰富了心房纤维化的可能发生机制,也为房颤的药物防治提供新的理论基础。在后续的实验中,我们将进行细胞水平实验,并且继续收集标本,收取足够的风心病阵发性房颤病例作为风心病阵发性房颤组。
[1] Savelieva I, Kourliouros A, Camm J. Primary and secondary prevention of atrial fibrillation with statins and polyunsaturated fatty acids: review of evidence and clinical relevance[J]. Naunyn Schmiedebergs Arch Pharmacol, 2010, 381(3): 207-219.
[2] Haim M, Hoshen M, Reges O, et al. Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non-valvular atrial fibrillation[J]. J Am Heart Assoc, 2015, 4(1): e001486.
[3] Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation[J]. J Am Coll Cardiol, 2008, 51(8): 802-809.
[4] Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology[J]. Pharmacol Rev, 2015, 67(2): 259-279.
[5] Shi S, Yoon DY, Hodge-Bell KC, et al. The aryl hydrocarbon receptor nuclear translocator (Arnt) is required for tumor initiation by benzo [a] pyrene[J]. Carcinogenesis, 2009, 30(11): 1957-1961.
[6] Pierre S, Chevallier A, Teixeira-Clerc F, et al. Aryl hydrocarbon receptor-dependent induction of liver fibrosis by dioxin[J]. Toxicol Sci, 2014, 137(1): 114-124.
[7] Bogoevska V, Wolters-Eisfeld G, Hofmann BT, et al. HRG/HER2/HER3 signaling promotes AhR-mediated Memo-1 expression and migration in colorectal cancer[J]. Oncogene, 2016 Dec 12. [Epub ahead of print]
[8] Kotecha D, Holmes J, Krum H, et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis[J]. Lancet, 2015, 384(9961): 2235-2243.
[9] Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population[J]. Am J Cardiol, 2013, 112(8): 1142-1147.
[10]Zhang L, Zhang N, Tang X, et al. Increased α-actinin-2 expression in the atrial myocardium of patients with atrial fibrillation related to rheumatic heart disease[J]. Cardio-logy, 2016, 135(3): 151-159.
[11]Li Y, Jian Z, Yang ZY, et al. Increased expression of connective tissue growth factor and transforming growth factor-beta-1 in atrial myocardium of patients with chronic atrial fibrillation[J]. Cardiology, 2013, 124(4): 233-240.
[12]何文聪, 李裕舒, 罗明华, 等. 心房颤动患者心房纤维化与缝隙连接重构的关系[J]. 中国病理生理杂志, 2008, 24(10): 1943-1947.
[13]Haarmann-Stemmann T, Bothe H, Abel J. Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways[J]. Biochem Pharmacol, 2009, 77(4): 508-520.
[14]Poormasjedi-Meibod MS, Salimi Elizei S, Leung V, et al. Kynurenine modulates MMP-1 and type-I collagen expression via aryl hydrocarbon receptor activation in dermal fibroblasts[J]. J Cell Physiol, 2016, 231(12): 2749-2760.
[15]Antos PA, Bachuta M, Hrabia A, et al. Expression of aryl hydrocarbon receptor 1 (AHR1), AHR1 nuclear translocator 1 (ARNT1) and CYP1 family monooxygenase mRNAs and their activity in chicken ovarian follicles followinginvitroexposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD)[J]. Toxicol Lett, 2015, 237(2): 100-111.
[16]Gostomska-Pampuch K, Ostrowska A, Kuropka P, et al. Protective effects of levamisole, acetylsalicylic acid, and α-tocopherol against dioxin toxicity measured as the expression of AhR and COX-2 in a chicken embryo model[J]. Histochem Cell Biol, 2017, 147(4): 523-536.
[17]Xue J, Zhao Q, Sharma V, et al. Aryl hydrocarbon receptor ligands in cigarette smoke induce production of interleukin-22 to promote pancreatic fibrosis in models of chronic pancreatitis[J]. Gastroenterology, 2016, 151(6): 1206-1217.
[18]Bacsi SG, Reisz-Porszasz S, Hankinson O. Orientation of the heterodimeric aryl hydrocarbon (dioxin) receptor complex on its asymmetric DNA recognition sequence[J]. Mol Pharmacol, 1995, 47(3): 432-438.
[19]Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD[J]. Biochim Biophys Acta, 2003, 1619(3): 263-268.
[20]Abbott BD, Schmid JE, Brown JG, et al. RT-PCR quantification of AHR, ARNT, GR, and CYP1A1 mRNA in craniofacial tissues of embryonic mice exposed to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin and hydrocortisone[J]. To-xicol Sci, 1999, 47(1): 76-85.
[21]Yaming P, Urs AB, Saxena A, et al. Roles of CYP1A1 and CYP2E1 gene polymorphisms in oral submucous fibrosis[J]. Asian Pac J Cancer Prev, 2016, 17(7): 3335-3340.
[22]Ghosh T, Gupta S, Bajpai P, et al. Association of CYP1A1, GSTM1, and GSTT1 gene polymorphism with risk of oral submucous fibrosis in a section of North Indian population[J]. Mol Biol Rep, 2012, 39(10): 9383-9389.
(责任编辑: 林白霜, 罗 森)
Expression and significance of aryl hydrocarbon receptor in atrial tissues of patients with atrial fibrillation
LEI Jian-ming, XIAO Hua, LI Qiang, WEI Xiao, GUO Jing-wen, XIAO Ming-han
(DepartmentofCardiology,TheFirstAffiliatedHospitalofChongqingMedicalUniversity,Chongqing400016,China.E-mail:xiaohua197408@163.com)
AIM: To investigate the expression of aryl hydrocarbon receptor (AhR) in atrial tissues of the patients with rheumatic heart disease (RHD), and the effects of AhR on rheumatic atrial fibrosis. METHODS: Right atrial specimens obtained from the patients with RHD requiring valve replacement surgery were divided into chronic atrial fibrillation (RHD+cAF,n=11) group and sinus rhythm (RHD+sinus rhythm,n=25) group. The patients with congenital heart disease (CHD) and sinus rhythm (CHD+sinus rhythm,n=12) who underwent heart surgery served as controls. The collagen volume fraction in the atrial specimens was examined by Masson’s trichrome staining. The protein expression and distribution of AhR, AhR nuclear translocator (ARNT) and CYP1A1 were detected by the methods of immunohistochemistry and Western blot. The mRNA expression of AhR, ARNT and CYP1A1 was detected by real-time fluorescence quantitative PCR. RESULTS: Compared with CHD+sinus rhythm group, the collagen content and the expression of AhR, ARNT and CYP1A1 were significantly increased in RHD+sinus rhythm group and RHD+cAF group. Compared with RHD+sinus rhythm group, the collagen content and the expression of AhR, ARNT and CYP1A1 were significantly increased in RHD+cAF group (P<0.05). CONCLUSION: The expression of AhR is correlated with the degree of fibrosis. The expression of AhR/ARNT/CYP1A1 is increased in atrial tissues of patients with RHD, suggesting that AhR/ARNT/CYP1A1 should be involved in atrial fibrosis of the patient with RHD.
Aryl hydrocarbon receptor; Atrial fibrillation; Fibrosis
1000- 4718(2017)05- 0826- 06
2016- 12- 19
2017- 03- 16
国家自然科学基金资助项目(No. 81300140)
R363
A
10.3969/j.issn.1000- 4718.2017.05.010
杂志网址: http://www.cjpp.net
△通讯作者 Tel: 023-89011565; E-mail: xiaohua197408@163.com

