进展性碘难治性分化型甲状腺癌患者阿帕替尼治疗后血清学与影像学指标变化*
张 鑫 王 宸 梁 军 林岩松
进展性碘难治性分化型甲状腺癌患者阿帕替尼治疗后血清学与影像学指标变化*
张 鑫①王 宸①梁 军②林岩松①
目的:评估阿帕替尼对于局部进展性碘难治性分化型甲状腺癌(radioactive iodine-refractory differentiated thyroid cancer,RAIR-DTC)中位随访7.9个月后的治疗效果。方法:随访中国医学科学院北京协和医院自2016年3月至2016年6月入组阿帕替尼治疗RAIR-DTC临床实验的受试者10例,从血清生化角度,甲状腺球蛋白(thyroglobulin,Tg)、甲状腺球蛋白抗体(thyroglobulin antibody,Tg-Ab)及影像学角度,靶病灶长度(target lesions,TL)观察阿帕替尼疗效及相关性,总结随访期间的不良事件(adverse event,AE)。结果:中位随访时间为7.9个月,在平均服用阿帕替尼6周内Tg呈快速下降趋势,平均下降60%,最大可达90%,提示该药物血清学疗效反应迅速,此后呈现稳定趋势,但停药3~14天即可观察到Tg的反弹趋势,升幅波动在4%~135%;TL在服用阿帕替尼平均8周内呈快速下降趋势,平均下降40%,最大可达60%,提示该药物快速的影像学疗效反应,此后呈稳定趋势,受停药影响不明显;Tg周变化速率(Tgvn)和TL周变化速率(TLvn)呈正相关[TLvn=0.17×Tgvn+0.50(r=0.56,P<0.05)];受试者因不良反应均有不同程度的剂量下调,剂量调整后AE于3~14天缓解,下调剂量至250 mg/d仍能有效控制病情。结论:阿帕替尼治疗进展性RAIR-DTC具有快速、持久的血清学及影像学反应,Tgvn和TLvn呈正相关,且Tg较TL更为敏感,应作为RAIR-DTC靶向治疗评估的客观指标。
阿帕替尼碘难治性分化型甲状腺癌酪氨酸激酶抑制剂血清学反应影像学反应
碘难治性分化型甲状腺癌(radioactive iodine-re⁃fractory differentiated thyroid cancer,RAIR-DTC)约占分化型甲状腺癌的30%,其表现为经131Ⅰ治疗后病灶不摄碘,治疗后反应不佳,十年生存率不足10%[1-2]。对于RAIR-DTC的诊治是全球关注的热点问题[3-5]。目前,其治疗方案主要针对不同部位、病理学特征采取手术、放疗及针对不同靶点的酪氨酸激酶抑制剂(tyrosine kinase inhibitors,TKI)等治疗手段[2-6]。索拉非尼和乐伐替尼为美国食品药品监督管理局(U.S.Food and Drug Administration,FDA)批准的可用于治疗RAIR-DTC的靶向药物[7-10],但昂贵的价格限制了其在中国的推广。阿帕替尼作为我国自主研发的以血管内皮生长因子受体-2(vascular endothelial growth factor receptor 2,VEGFR-2)为靶点的小分子TKI[11-14],目前已被我国食品药品监管管理总局(CFDA)批准上市,用于标准化疗失败后的胃癌治疗[15-16],并相继开展治疗乳腺癌、食管癌、结直肠癌、肝癌、非小细胞肺癌(non-small cell lung cancer,NSCLC)等临床试验[17-21]。本课题组初步研究表明,阿帕替尼可安全用于RAIR-DTC,且在8周治疗后从血清学及结构影像学角度证实其快速有效、客观缓解率(objective response rate,ORR)较高[22]。本研究中位随访阿帕替尼治疗RAIR-DTC 7.9个月的血清学及影像学变化趋势。
1 材料与方法
1.1 临床资料
本研究已通过中国医学科学院北京协和医院伦理委员会的审批。所有患者在入组前均被告知研究的相关利弊及风险,并签署知情同意书(伦理批号:北京协和医院HS-970)。随访阿帕替尼治疗局部进展性碘难治性甲状腺癌Ⅱ期临床试验自2016年3月至2016年6月入组的10名受试者,分析其由入组至2016年12月31日期间的病情变化情况。所有患者均符合本试验标准,共18个靶病灶,平均年龄54.9(32~76)岁,男女比例为1∶1。目前,共有7例在访,1例因不良反应停药7 d,停药后肺部出现新病灶于第16周评定为PD,1例于第7周因不能耐受药物不良反应脱落,1例于第22周因肺部感染住院而停药,第24周达总生存期(overall survival,OS)终止随访,随访时间最长291d,最短111 d,平均224 d。基本资料见表1。1.1.1入组标准1)年龄≥18岁,性别不限;2)乳头状、滤泡状、Hurthle细胞及低分化癌局部晚期或转移性分化型甲状腺癌(differentiated thyroid cancer,DTC),至少有1个经治疗的可测量病灶,计算机断层显像(computed tomography,CT)扫描长径≥10 mm,符合实体瘤疗效评价标准1.1(response evaluation criteria in solid tumors 1.1,RECIST 1.1)的要求。包括:完全缓解(complete response, CR)、部分缓解(partial response,PR)、疾病稳定(stable disease,SD)、疾病控制率(disease control rate,DCR)和ORR等[23];3)在入组之前14个月内出现疾病进展(必须使用RECIST 1.1作为疾病进展的评估依据);4)放射性碘难治(满足下述条件之一):①靶病灶(target lesions,TL)在放射性碘治疗中完全丧失摄碘能力;②患者12个月内接受单次放射碘治疗(≥3.7 GBq)且靶病灶疾病进展;③患者每2次放射性碘治疗时间间隔<12个月,剂量≥3.7 GBq,至少有1次碘治疗后超过12个月疾病进展;④累计接受放射性碘治疗剂量≥22.2 GBq。
1.1.2 排除标准1)DTC外的其他甲状腺癌组织学亚型(如髓样癌、淋巴瘤或肉瘤);2)6个月内使用过VEGFR-TKI小分子药物,如凡德他尼(vandetanib)、卡巴唑替尼(cabozantinib)、乐伐替尼(lenvatinib)、舒尼替尼(sunitinib)及索拉非尼(sorafenib)等治疗的患者;3)患有高血压,经降压药物治疗无法降至正常范围者(收缩压>140 mmHg、舒张压>90 mmHg),患有≥Ⅱ级的冠心病、心律失常(包括QTc间期延长男性>450 ms,女性>470 ms)及心功能不全;4)具有影响口服药物吸收的多种因素(如无法吞咽、恶心呕吐、慢性腹泻和肠梗阻等);5)具有胃肠道出血风险的患者不可入组,包括下列情况:①有活动性消化溃疡病灶,且大便潜血(++);②3个月内有黑便、呕血病史者;6)凝血功能异常(INR>1.5倍正常上限(upper lim⁃it of normal,ULN)、APTT>1.5×ULN),具有出血倾向者;7)既往接受化疗抗甲状腺癌治疗(允许使用低剂量化疗进行放射增敏)或沙利度胺及其衍生物治疗;8)怀孕或哺乳期妇女。
1.2 方法
阿帕替尼750 mg,每天1次,口服。每4周定义为1个治疗周期。每个治疗周期允许停药≤2次。允许下调2次剂量至250 mg,但不允许上调剂量。
1.3 疗效检测
所有患者均于入组时检测甲状腺球蛋白(thyro⁃globulin,Tg)、甲状腺球蛋白抗体(thyroglobulin antibody,Tg-Ab)水平及靶病灶CT,并于服药后定期随访。前2个周期,每2~4周复查Tg和Tg-Ab,每4周复查CT;2个周期后,每4~8周复查Tg及Tg-Ab,每8周测量靶病灶,并计算Tg同比下降率。Tg同比下降率为:(Tg-Tg基线)/ Tg基线;靶病灶同比缩小率为:(TL-TL基线)/TL基线。此外,计算Tg平均周变化速率,定义为相邻两次Tg测量值之差除以时间间隔,公式为:Tgvn=[Tgn-Tg(n-1)]/间隔时间;同理,计算靶病灶周平均变化速率,公式为:TLvn=[TLn-TL(n-1)]/间隔时间,以初步评估患者血清学及影像学变化情况。
2 结果
2.1Tg同比变化率
10例受试者中共有8例Tg水平可用于评估,另2例因TgAb阳性(TgAb>100.00 IU/mL)影响Tg测量,故未纳入评价。平均服用阿帕替尼6周内Tg呈快速下降趋势,平均下降60%,最大可达90%,此后呈稳定趋势,中位迅速起效时间为6周;停药3~14 d Tg即可上浮4%~135%,恢复用药后可降至停药前水平;变化如图1,2。
2.2 基于RECIST 1.1评估标准的病灶长度变化
选取18个靶病灶应用RECISIT 1.1标准进行疗效评价[23]。平均服用阿帕替尼8周内TL呈快速下降趋势,平均下降40%,最大可达60%,此后呈稳定趋势,受停药影响不明显;其中,70%(7/10)患者TL变化在8周时趋于稳定;停药后TL未见明显波动,具体趋势如图3。从疗效评估结果分析,6例受试者目前仍呈PR;1例转为SD,1例因AE于第32周出组,1例因肺部感染死亡,1例因出现新病灶评定为PD。截至2016年12月31日,本试验DCR为90%,ORR为70%,中位无进展随访时间为222.5 d(图3,4)。
2.3 安全性
截至最后一次随访,10例受试者均出现Ⅲ级及以上不良事件:6例受试者出现手足皮肤反应(hand-foot skin reaction,HFSR)共计16例次,为最常见AE;高血压、胃痛分列2、3位。1例受试者出现Ⅳ级低钙血症。受试者用药剂量均有所下调,截至最后一次随访,7例服用阿帕替尼剂量降至500 mg/d,3例降至250 mg/d。停药3~14 d后即可明显改善。经剂量下调、对症处理后,患者均能耐受药物不良反应。另有1例受试者因肺部感染住院治疗,与药物不良反应无关(表2)。
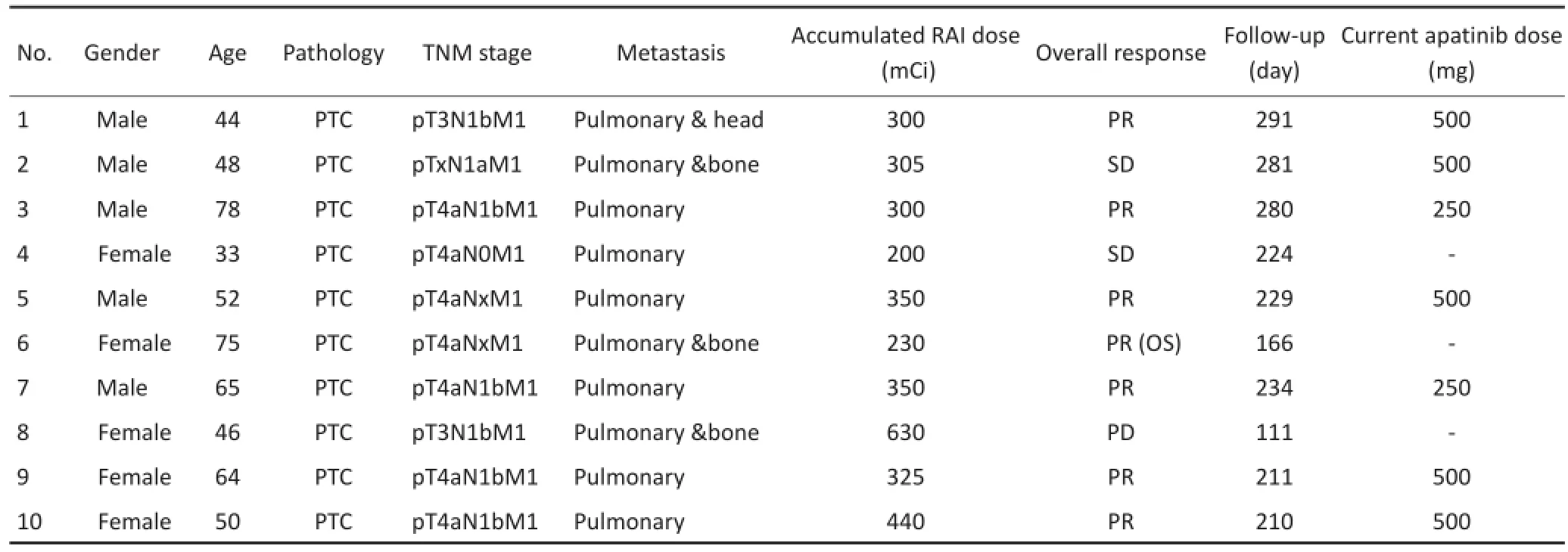
表1 患者基本资料Table1 Characteristics of enrolled patients
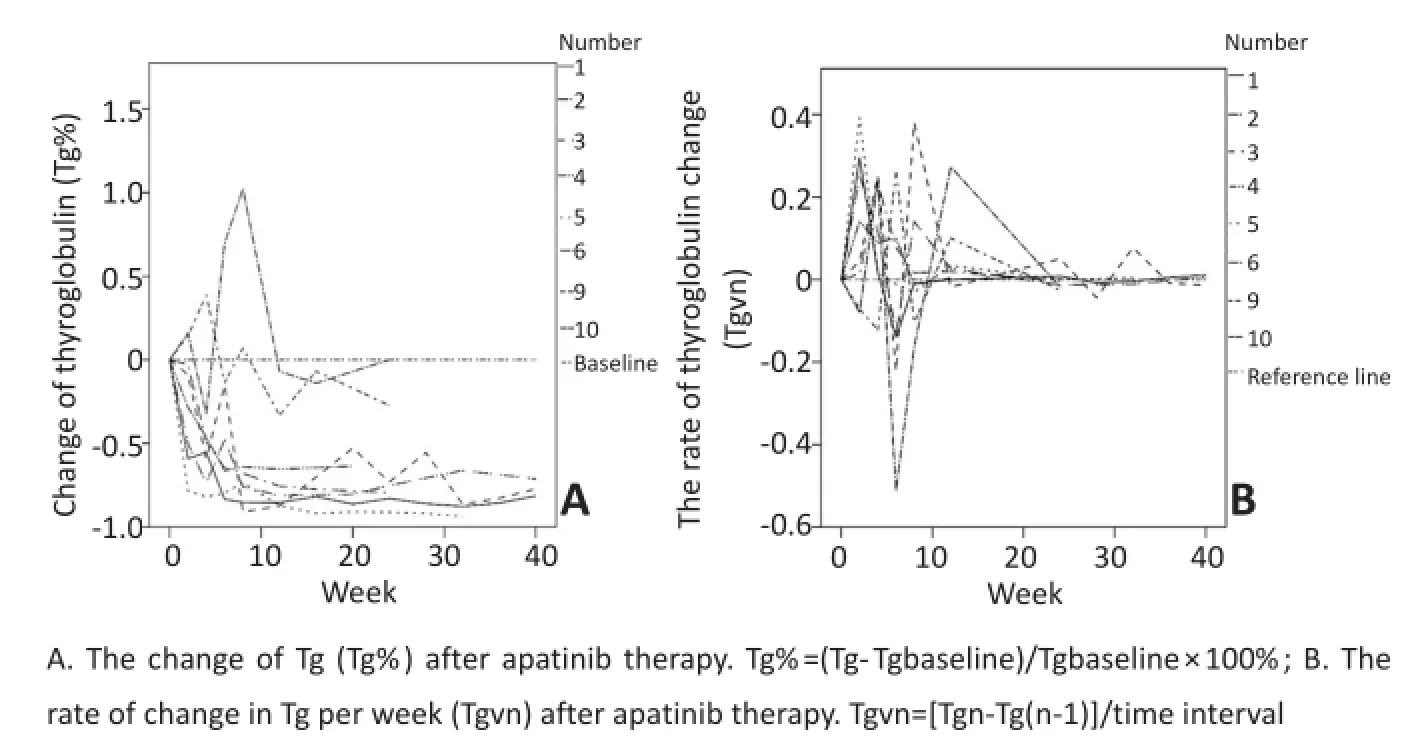
图1 甲状腺球蛋白(Tg)变化Figure1 Tg change
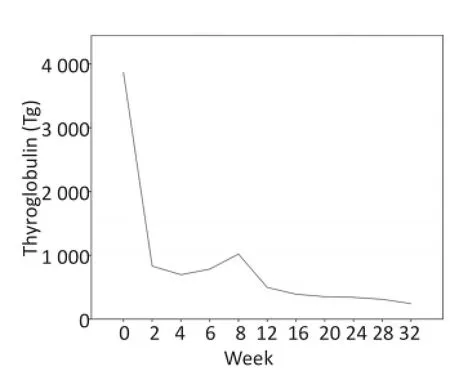
图2 典型病例甲状腺球蛋白(Tg)变化Figure2 Change of Tg in a typical case
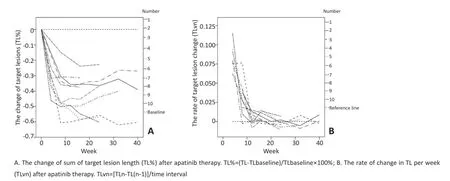
图3 靶病灶长度(TL)变化Figure3 Target lesion length(TL)
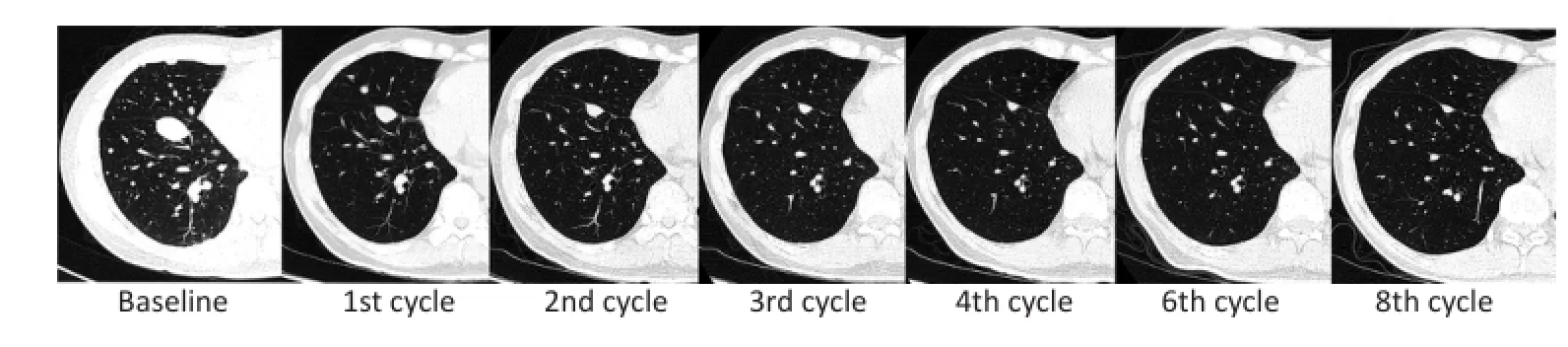
▶图4典型病例病灶变化Figure4 The change of the target lesions in CT of a typical case
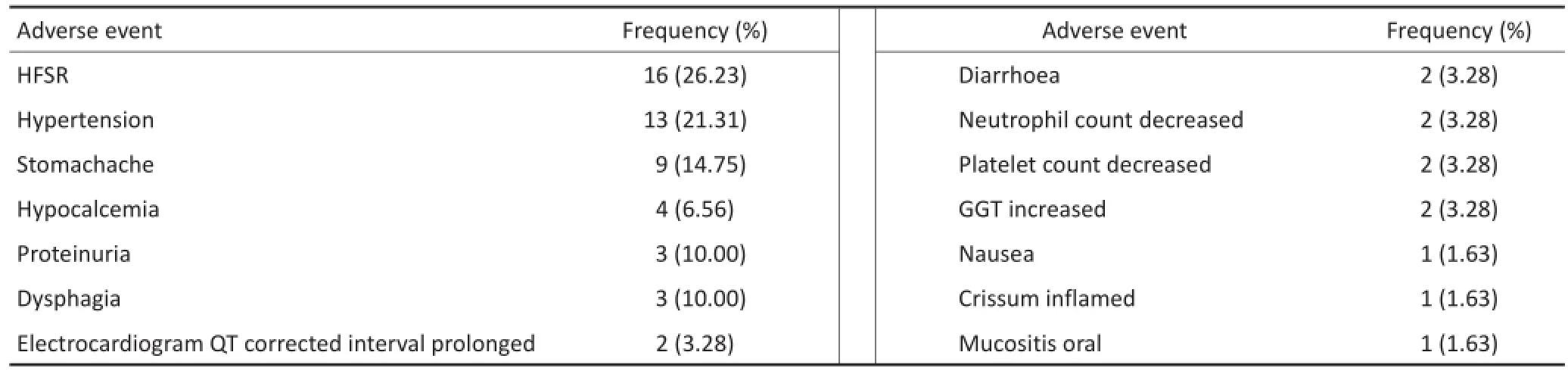
表2 不良事件发生情况Table2 Adverse events
3 讨论
目前,RECIST 1.1标准是评估肿瘤靶向药物治疗效果的主要标准。本试验表明,服用阿帕替尼早期TL呈快速下降趋势,反映其快速、持久的影像学疗效。但监测TL需调动较多的人力、物力,监测周期长,患者在接受影像学检查时需要接受一定辐射剂量,且不能反映停药时阿帕替尼对于肿瘤控制作用的影响。
占甲状腺癌90%以上的DTC部分保留了甲状腺滤泡细胞的功能,如合成Tg的能力。因此,血清Tg也成为评估DTC术后、131Ⅰ治疗效果及预后的重要血液生化特异性指标,其检测手段较比TL监测更为方便、快捷、经济[24-25]。关于TKI治疗RAIR-DTC后Tg的监测已有报道,如服用乐伐替尼4周后Tg即迅速下降,在达到疾病控制的受试者(PR和SD)Tg降幅达65.5%,提示其从血清学角度对肿瘤的快速控制[26-27]。本研究发现平均服用阿帕替尼6周内Tg呈快速下降趋势,平均下降约60%,这与乐伐替尼所提示的快速血清学疗效相一致。在此基础上,本研究进一步探索Tg变化在监测阿帕替尼疗效中的应用价值以及Tg与TL变化速率的关系。本研究提示,停服阿帕替尼约8个半衰期(t1/2,约9 h)即可出现Tg回升,说明其可灵敏地反映肿瘤在失去阿帕替尼控制后快速反弹,提示Tg作为阿帕替尼治疗RAIR-DTC疗效的监测指标具较高敏感性。但因缺少RAIR-DTC患者服用阿帕替尼的药代动力学证据,尚需深入研究[11]。本研究首次观察到阿帕替尼治疗RAIRDTC中Tgvn和TLvn呈正相关[TLvn=0.17×Tgvn+0.50(r= 0.56,P<0.05)],提示Tg作为肿瘤标志物可以辅助预测TL变化情况。综合阿帕替尼服用过程中Tg及TL变化规律及停药情况对相关指标的影响,本研究认为Tg能更敏感地反映阿帕替尼对肿瘤的控制作用。监测Tg变化有利于早期预测及评估阿帕替尼对RAIR-DTC的治疗效果,筛选获益人群;停药后密切的Tg监测更有助于及早发现病情变化,为采用其他有效治疗争取时间(图5)。
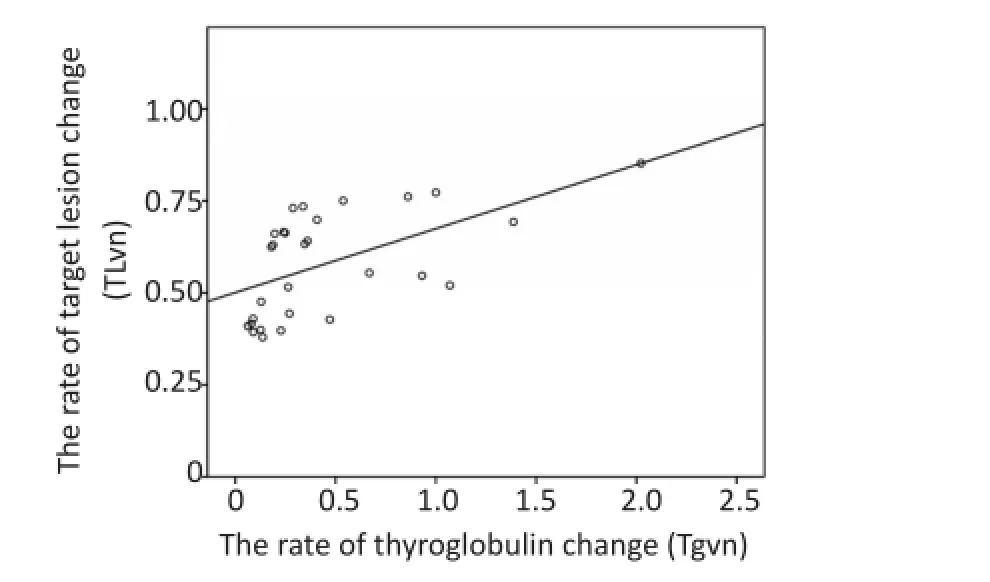
图5 靶病灶变化速率(TLvn)和甲状腺球蛋白变化速率(Tgvn)散点图Figure5 Scatter plot of target lesion change rate(TLvn)and thyroglob‐ulin change rate(Tgvn)
截至2016年12月31日,本研究中位随访时间为7.9个月,虽然低于索拉非尼治疗RAIR-DTC中位无进展生存时间(median progression free survival,mPFS)10.8个月,但高于其安慰剂组5.8个月[7]。此外,目前本试验最长无进展随访时间为10.4个月,接近索拉非尼PFS。提示阿帕替尼对于控制RAIRDTC具有良好的有效性。研究表明,TKI靶向治疗效果和不良反应有关[28]。在阿帕替尼治疗中,因发生Ⅲ级及以上AE,受试者用药剂量均有不同程度的调整,减量后仍能维持SD,提示有待扩大样本量进一步摸索阿帕替尼治疗RAIR-DTC的最佳剂量,以兼顾安全性及有效性。本试验样本量较少、缺乏停药对Tg影响的系列数据(停药起止测定Tg水平)等,在后续Ⅲ期临床试验中将进一步观察并报告。
综上所述,本单臂前瞻性研究提示,在中位随访7.9个月时间内阿帕替尼治疗进展性RAIR-DTC具有快速、持久的血清学及影像学反应,血清Tg和靶病灶的变化呈正相关,且Tg较CT影像学评估更为敏感,可作为RAIR-DTC靶向治疗评估的客观指标和CT等影像学评估的重要补充。
[1]Martin S,Marcia B,Rosella E,et al.Definition and management of radioactive iodine‐refractory differentiated thyroid cancer[J].Lanc Diabe Endocrinol,2014,2(5):356‐358.
[2]Schlumberger M,Chougnet C,Baudin E,et al.Refractory thyroid cancers[J].Presse Medi,2011,40(12):1189‐1198.
[3]Dan Z,Xiaona J,Fang L,et al.Integrin alpha(v)beta(3)imaging of radioactive iodine‐refractory thyroid cancer using Tc‐99m‐3PRGD2 [J].J Nucl Medi,2012,53(12):1872‐1877.
[4]Sherman EJ,Su YB,Lyall A,et al.Evaluation of romidepsin for clini‐cal activity and radioactive iodine reuptake in radioactive iodine‐re‐fractory thyroid carcinoma[J].Thy Offic J Am Thy Associ,2013,23 (5):593‐599.
[5]Mcfarland DC,Misiukiewicz KJ.Sorafenib in radioactive iodine‐re‐fractory well‐differentiated metastatic thyroid cancer[J].Oncot Ther,2014,7:1291‐1299.
[6]Johnson LN.Protein kinase inhibitors:contributions from structure to clinical compounds[J].Quart Rev Bio,2009,42(1):1‐40.
[7]Brose MS,Nutting CM,Jarzab B,et al.Sorafenib in radioactive io‐dine‐refractory,locally advanced or metastatic differentiated thy‐roid cancer:a randomised,double‐blind,phase 3 trial[J].Lancet, 2014,384(9940):319‐328.
[8]Marotta V,Ramundo V,Camera L,et al.Sorafenib in advanced io‐dine‐refractory differentiated thyroid cancer:efficacy,safety and exploratory analysis of role of serum thyroglobulin and FDG‐PET[J]. Clin Endocri,2013,78(5):760‐767.
[9]Schneider TC,Abdulrahman RM,Corssmit EP,et al.Long‐term anal‐ysis of the efficacy and tolerability of sorafenib in advanced radio‐iodine refractory differentiated thyroid carcinoma:final results of a phaseⅡtrial[J].Euro J Endocri,2012,167(167):643‐650.
[10]Nair A,Lemery SJ,Yang J,et al.FDA Approval summary:lenvatinib for progressive,radio‐iodine‐refractory differentiated thyroid can‐cer[J].Clin Cancer Res,2015,21(23):5205.
[11]Zhang H.Apatinib for molecular targeted therapy in tumor[J].Drug Design Deve Ther,2015,9:6075‐6081.
[12]Ding J,Chen X,Gao Z,et al.Metabolism and pharmacokinetics of novel selective vascular endothelial growth factor receptor‐2 inhibi‐tor apatinib in humans[J].Drug Metabol Dispo,2013,41(6):1195‐1210.
[13]Scott AJ,Messersmith WA,Jimeno A.Apatinib:a promising oral an‐tiangiogenic agent in the treatment of multiple solid tumors[J]. Drugs of Today,2015,51(4):223‐229.
[14]Zhang C,Tan C,Ding H,et al.Selective VEGFR inhibitors for antican‐cer therapeutics in clinical use and clinical trials[J].Cur Pharm Desi, 2012,18(20):2921‐2935.
[15]Zhang S.Problematic analysis and inadequate toxicity data in phaseⅢapatinib trial in gastric Cancer[J].J Clin Oncol Offic J Am Soci Clin Oncol,2016,34(31):3821.
[16]Li J,Qin S,Xu J,et al.Apatinib for chemotherapy‐refractory ad‐vanced metastatic gastric cancer:results from a randomized,place‐bo‐controlled,parallel‐arm,phaseⅡtrial[J].J Clin Oncol Offic J Am Soci Clin Oncol,2013,31(26):3219‐3225.
[17]Ding L,Li QJ,You KY,et al.The use of apatinib in treating non small‐cell lung cancer:case report and review of literature[J].Medic, 2016,95(20):e3598.
[18]Peng S,Zhang Y,Peng H,et al.Intracellular autocrine VEGF signaling promotes EBDC cell proliferation,which can be inhibited by Apa‐tinib[J].Cancer Letters,2016,373(2):193‐202.
[19]Veer ET,Mohammad NH,Valkenhoef GV,et al.Second‐and third‐line systemic therapy in patients with advanced esophagogastric cancer:a systematic review of the literature[J].Cancer Metasta Rev,2016,35(3):439‐456.
[20]Zhou N,Liu CM,Hou H L,et al.Response to apatinib in chemothera‐py‐failed advanced spindle cell breast carcinoma[J].Oncotarget, 2016,7(44):72373‐72379.
[21]Langer CJ,Mok T,Postmus PE.Targeted agents in the third‐/fourth‐line treatment of patients with advanced(stageⅢ/Ⅳ)non‐small cell lung cancer(NSCLC)[J].Cancer Treat Revi,2013,39(3):252‐260. [22]Lin YS,Wang C,Li H,et al.The preliminary report about the efficacy and safety evaluation of apatinib in progressive radioactive iodine‐refractory differentiated thyroid cancer within 8 weeks[J].China Oncol,2016,26(9):721‐726.[林岩松,王宸,李慧,等.甲磺酸阿帕替尼治疗进展性碘难治性甲状腺癌的短期疗效及安全性初步报告[J].中国癌症杂志,2016,26(9):721‐726.]
[23]Bogaerts J.New response evaluation criteria in solid tumours:re‐vised RECIST guideline(version 1.1)[J].Gan to Kagaku Ryoho Can‐cer&Chemotherapy,2009,36(13):2495‐2501.
[24]Haugen BR.2015 American thyroid association management guide‐lines for adult patients with thyroid nodules and differentiated thy‐roid cancer:what is new and what has changed[J]?Thyroid,2016, 26(1):1‐133.
[25]Perros P,Boelaert K,Colley S,et al.British thyroid association guide‐lines for the management of thyroid cancer[J].Clin Endocri,2014, 81(Suppl 1):1‐122.
[26]Maomei R,Shen Y,Chen L,et al.RECIST 1.1 and serum thyroglobu‐lin measurements in the evaluation of responses to sorafenib in pa‐tients with radioactive iodine‐refractory differentiated thyroid car‐cinoma[J].Oncol Lett,2013,6(2):480‐486.
[27]Werner RA,Lückerath K,Schmid JS,et al.Thyroglobulin fluctuations in patients with iodine‐refractory differentiated thyroid carcinoma on lenvatinib treatment‐initial experience[J].Sci Rep,2016,6:28081.
[28]Izumi K,Itai S,Takahashi Y,et al.Predictive factor and antihyperten‐sive usage of tyrosine kinase inhibitor‐induced hypertension in kid‐ney cancer patients[J].Oncol Lett,2014,8(1):305‐308.
(2017‐02‐15收稿)
(2017‐03‐22修回)
(编辑:孙喜佳校对:郑莉)
Follow-up study on biochemical and structural response in progressive radioactive iodine-refractory differentiated thyroid cancer patients treated with apatinib
Xin ZHANG1,Chen WANG1,Jun LIANG2,Yansong LIN1
1Department of Nuclear Medicine,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences,Beijing 100730,Chi‐na;2Department of Oncology,Peking University International Hospital,Beijing 102206,China
Yansong LIN;E‐mail:LinYS@pumch.cn
Objective:To evaluate the biochemical and structural changes of apatinib in patients with progressive radioactive iodine‐re‐fractory differentiated thyroid cancer(RAIR‐DTC).Methods:The participants(n=10)were followed up since March 2016.Treatment ef‐fect was evaluated in using both biochemical[thyroglobulin(Tg)and thyroglobulin antibody(Tg‐Ab)]and structural responses(target lesions,TL).Adverse events were also recorded over time.Results:The median follow‐up was 7.9 months.The Tg level declined rapid‐ly within 6 weeks after apatinib treatment,and the average decline ranged from 60%to 90%,indicating the immediate biochemical re‐sponse of apatinib in progressive RAIR‐DTC.The Tg level tended to stabilize thereafter.However,the Tg level rebounded by 4%–135% when withdrawal was performed for 3–14 days.The number of TLs decreased rapidly within 8 weeks,and the average decreased ranged from 40%to 60%,indicating the presence of rapid structural responses.Thereafter,the number of TLs continued to stabilize. TLs,in contrast to Tg,were not significantly affected by drug withdrawal.The rate of change in Tg(Tgvn)was positively correlated with the rate of change in TL(TLvn)[TLvn=0.17×Tgvn+0.50(r=0.56,P<0.05)].The apatinib dose was adjusted due to adverse events,which could be relieved after 3 to 14 days of withdrawal.Apatinib can effectively control the disease even at a reduced dose of 250 mg/d. Conclusion:Apatinib treatment showed a fast and sustainable biochemical and structural responses.Tg could be regarded as an objec‐tive indicator.Tgvn is positively correlated with TLvn,and the response of Tg is more sensitive than that of TLs.
apatinib,radioactive iodine‐refractory differentiated thyroid cancer,tyrosine kinase inhibitor,biochemical response,struc‐tural response

10.3969/j.issn.1000-8179.2017.08.172
张鑫专业方向为甲状腺癌分子影像学诊断及治疗。E-mail:zhangx716@126.com
①中国医学科学院北京协和医院核医学科(北京市100730);②北京大学国际医院肿瘤内科
*本文课题受国家自然科学基金项目(编号:81571714)资助
林岩松LinYS@pumch.cn
This work was supported by National Natural Science Foundation of China(No.81571714)

