氯虫苯甲酰胺亚致死剂量对甜菜夜蛾主要解毒酶活性与生长繁殖的影响
陈羿渠,向兴,贡常委,王学贵
(四川农业大学农学院无公害农药研究实验室,成都 611130)
氯虫苯甲酰胺亚致死剂量对甜菜夜蛾主要解毒酶活性与生长繁殖的影响
陈羿渠,向兴,贡常委,王学贵
(四川农业大学农学院无公害农药研究实验室,成都 611130)
【目的】甜菜夜蛾(Spodoptera exigua)是一种杂食性害虫,在不同环境、药物等选择压力下表现出不同的生长发育特点。氯虫苯甲酰胺是一种新型广谱的鱼尼丁受体杀虫剂,对鳞翅目害虫杀虫活性强。本研究旨在探究氯虫苯甲酰胺亚致死剂量对甜菜夜蛾幼虫3种主要解毒酶——羧酸酯酶(CarE)、谷胱甘肽S-转移酶(GSTs)和多功能氧化酶(MFOs)活性以及对种群繁殖的影响。【方法】采用饲料混毒法测定氯虫苯甲酰胺对SE-Lab品系、SE-Sel品系的毒力,SE-Sel品系由SE-Lab品系经亚致死剂量LC25连续汰选6代得到;通过浸叶法测定磷酸三苯酯(TPP)、顺丁烯二酸二乙酯(DEM)、胡椒基丁醚(PBO)3种酶抑制剂与氯虫苯甲酰胺协同对SE-Lab和SE-Sel品系的毒力增效作用,提前12 h让试虫取食浸渍过酶抑制剂的叶片,对照组取食用0.1% TritonX-100浸渍后的叶片,再分别测定氯虫苯甲酰胺对使用酶抑制剂与未使用酶抑制剂试虫的毒力;在冰上解剖试虫的中肠和脂肪体,并通过离体酶活性测定,分析氯虫苯甲酰胺亚致死剂量与酶抑制剂对甜菜夜蛾体内的3种代谢解毒酶活力的影响;通过记录试虫各个年龄阶段的生长、死亡、产卵量等数据,参照两性生命表理论分析SE-Lab和SE-Sel品系的两性生命表参数差异。【结果】在3种酶抑制剂中PBO增效作用最强,其对甜菜夜蛾SE-Sel品系和SE-Lab品系对氯虫苯甲酰胺的毒力增效比分别为1.58和1.69。在氯虫苯甲酰胺亚致死剂量连续汰选下,甜菜夜蛾体内3种解毒酶活性均被诱导上升,其中MFOs酶活力上升最显著,SE-Sel品系中肠和脂肪体的MFOs活性相对于SE-Lab品系分别提高了2.07和2.10倍,而经氯虫苯甲酰胺亚致死剂量再次诱导的SE-Sel试虫的MFOs活性亦较SE-Lab品系上升4.02和3.44倍;在使用了酶抑制剂后3种解毒酶酶活力均有下降,其中MFOs活性下降最多,其酶比活力仅为未使用酶抑制剂处理的42.3%—44.8%。与SE-Lab品系相比,SE-Sel品系成虫的产卵前期和总产卵前期变长,而产卵量减少;SE-Sel品系的内禀增长率(r)、周限增长率(λ)和净增殖率(R0)均显著小于SE-Lab品系,SE-Lab品系与SE-Sel品系的r分别为0.18和0.16 d-1,λ为1.20和1.17 d-1, R0为358.42和203.12 d-1。尽管SE-Sel品系的平均世代周期(T)更长,但是与SE-Lab品系无显著差异。【结论】MFOs可能为甜菜夜蛾对氯虫苯甲酰胺解毒代谢过程中的主要解毒酶,在其后续抗性形成中起主要作用;甜菜夜蛾在氯虫苯甲酰胺亚致死剂量作用下,世代周期延长,繁殖力降低,种群增长减缓,氯虫苯甲酰胺亚致死剂量对甜菜夜蛾有持续控制作用。
甜菜夜蛾;氯虫苯甲酰胺;亚致死剂量;增效剂;多功能氧化酶;两性生命表
0 引言
【研究意义】甜菜夜蛾(Spodoptera exigua)是一种世界范围分布的重要农业害虫,可取食多种经济作物[1-2]。氯虫苯甲酰胺是美国杜邦公司研发的新一代二酰胺类超高效杀虫剂,通过激活昆虫鱼尼丁受体,过度释放细胞内储存的钙离子,导致昆虫瘫痪抽搐,丧失行动能力,进而死亡[3-4]。由于其对多种鳞翅目害虫防控效果明显,在田间大量使用,加速了靶标害虫抗药性的产生[5]。目前,甜菜夜蛾对氯虫苯甲酰胺具有抗性风险,个别种群已达到高抗性[6]。昆虫除了直接被杀虫剂杀死外,随着施药时间的增长,药剂部分降解至剂量不足以杀死昆虫个体的亚致死剂量,该剂量仍可引起害虫生态学、生殖力的变化、抗药性的发展、药剂代谢能力变动等[7-8],CHI[9]创建了年龄-龄期两性生命表理论,该理论在外界条件对昆虫种群生长繁殖影响的研究中越来越多地被运用[10-11]。【前人研究进展】昆虫产生抗药性的主要机理包括表皮穿透速率降低、对杀虫剂解毒代谢作用增强(代谢解毒酶主要包括多功能氧化酶(MFOs)、羧酸酯酶(CarE)及谷胱甘肽S-转移酶(GSTs))、靶标位点敏感性下降以及昆虫行为发生改变等[12]。在甜菜夜蛾的研究中,有报道溴氰虫酰胺亚致死剂量对甜菜夜蛾幼虫体内解毒酶活性有不同程度的抑制作用[13]。WANG等[14]采用多杀菌素亚致死剂量汰选所得的抗性品系,其多功能氧化酶活性是敏感品系的5.2倍;在酶抑制剂的作用下,对氰氟虫腙高抗的甜菜夜蛾种群 CarE的增效作用明显高于MFOs与GSTs[15]。除甜菜夜蛾外,杀虫剂亚致死剂量对昆虫解毒酶影响在多种鳞翅目昆虫中均有报道,如小菜蛾(Plutella xylostella)、二化螟(Chilo suppressalis)、棉铃虫(Helicoverpa armigera)等[16-18]。目前关于食物、温度等外界条件对两性生命表基本参数影响的报道较多[19-22],近年也有通过两性生命表研究杀虫剂影响昆虫生长繁殖的相关报道,ESMAEILY等[23]分别用阿维菌素、吡虫啉、二嗪磷和吡蚜酮,以及白花牛角瓜(Calotropis procera)乙醇提取物对烟粉虱(Bemisia tabaci)进行处理,分析其两性生命表数据,发现吡蚜酮与白花牛角瓜乙醇提取物处理的试虫内禀增长率(r)、周限增长率(λ)均小于其他药剂处理;JAFARBEIGI等[24]利用两性生命表比较了4种植物乙醇提取物与吡蚜酮亚致死剂量对烟粉虱的生长繁殖的影响,筛选出两种对烟粉虱防治效果理想的植物提取物;RAHMANI等[25]研究了噻虫嗪亚致死剂量对异多瓢虫(Hippodamia variegata)两性生命表参数的影响,结果表明噻虫嗪的使用不利于捕食性瓢虫的生长繁殖,其亚致死剂量对3龄幼虫的发育历期、成虫寿命和产卵量有显著影响;SCHNEIDER等[26]研究发现,将草甘膦浸泡后的草蛉(Chrysoperla externa)卵与正常卵进行两性生命表参数统计,经过一个生长周期后,发现草甘膦处理组的成虫繁殖力较对照组明显下降。【本研究切入点】经氯虫苯甲酰胺亚致死剂量连续诱导甜菜夜蛾,通过增效剂试验和对解毒代谢酶活的测定,初步确定甜菜夜蛾代谢氯虫苯甲酰胺的解毒酶类型;采用两性生命表研究克服了传统生命表中仅突出雌虫对种群增长的贡献,而忽略雄虫对昆虫种群发展的贡献[11,27]。试验对SE-Lab和SE-Sel品系采用两性生命表进行种群分析,研究了氯虫苯甲酰胺亚致死剂量对甜菜夜蛾种群参数的影响,此研究未见报道。【拟解决的关键问题】明确抗性形成相关的解毒代谢酶类型后,为表达相关解毒代谢酶的基因深入分析打下基础;根据生命表的研究结果,了解氯虫苯甲酰胺对甜菜夜蛾生长发育等方面的影响,为甜菜夜蛾田间防控提供理论支持和数据支撑。
1 材料与方法
试验于 2016年在四川农业大学农学院无公害农药研究实验室完成。
1.1 供试虫源及饲养
甜菜夜蛾SE-Lab品系(SE-Lab):2011年从中国农业大学昆虫生理生化与分子毒理学实验室引种连续饲养至今,实验室饲养条件为温度(25±1)℃,相对湿度(65±5)%,光周期14L﹕10D。初孵幼虫在含有人工饲料的指形管内(直径2.0 cm,高8.0 cm)饲养至化蛹,将蛹置于 0.5%次氯酸钠溶液中浸泡消毒后,放入养虫笼内保湿培养待其羽化,成虫羽化后饲喂10%蜂蜜水为其产卵提供营养,养虫笼内放入有褶皱的硫酸纸供成虫产卵,定期收集卵块,并用0.5%次氯酸钠溶液对卵块浸泡消毒后晾干,放入封口袋中,待幼虫孵化。
甜菜夜蛾SE-Sel品系(SE-Sel):以SE-Lab品系为基础品系,4龄幼虫时以饲料混毒法饲喂含LC25(SE-Lab品系亚致死剂量)氯虫苯甲酰胺人工饲料,48 h后继续饲喂新鲜饲料,连续汰选6代后继续饲养,饲养方法同SE-Lab品系。
1.2 供试药剂与仪器
95%氯虫苯甲酰胺原药(美国杜邦公司);磷酸三苯酯(TPP)、顺丁烯二酸二乙酯(DEM)、胡椒基丁醚(PBO)(上海aladdin试剂公司);TritonX-100、α-乙酸萘酯(α-NA)、α-萘酚、固蓝B盐、对硝基苯甲醚、1-氯-2, 4-二硝基苯(CDNB)(成都艾科达化学试剂有限公司);二硫苏糖醇(DTT)、苯甲基磺酰氟(PMSF)、还原型谷胱甘肽、NADPH、牛血清蛋白(北京索莱宝科技有限公司);毒扁豆碱(eserine)(Sigma-Aldrich公司);乙二胺四乙酸二钠盐(EDTANa2)、十二烷基硫酸钠(SDS)、考马斯亮蓝G250、丙三醇、85%磷酸、NaH2PO4、Na2HPO4、NaOH、37%盐酸(成都市科龙化工试剂厂)。
紫外可见分光光度计UV-3000型(上海美谱达仪器有限公司)、全自动酶标仪680型(Bio-Rad公司)、台式高速大容量冷冻离心机 5810型(Eppendorf 公司)。
1.3 生物测定
饲料混毒法:参照余慧灵等[13]方法,用丙酮将配置氯虫苯甲酰胺1 g·L-1母液,用0.1% TritonX-100将母液稀释成梯度浓度,取1 mL稀释后的药液加入至50 g人工饲料中混匀,试验工作浓度为0.005、0.01、0.02、0.04、0.08、0.16 µg·g-1,并设0.1% TritonX-100水溶液为空白对照,在细胞培养板中加入2.5 g已配好的混毒饲料,接入长势均匀的甜菜夜蛾4龄初期幼虫(试虫体重范围 3.78—4.65 mg/头),每个浓度处理15头,每个处理3次重复。
浸叶法:参照LAI等[6]方法,将已经配置好的氯虫苯甲酰胺1 g·L-1母液用0.1% TritonX-100稀释成梯度浓度,试验工作浓度为0.2、0.4、0.6、0.8、1.0、1.2 mg·L-1,后将甘蓝叶片(Φ=5.0 cm)在药液中浸泡20 s,待其晾干后放入塑料培养皿(Φ=9.0 cm)中,每个培养皿接入4龄初期甜菜夜蛾幼虫(试虫体重范围3.78—4.65 mg/头)5头,每个浓度处理15头,每个处理重复3次,用0.1% TritonX-100处理的叶片做对照。解毒酶抑制剂测定时,用丙酮提前将酶抑制剂配制为10 g·L-1的母液,再用0.1% TritonX-100将母液稀释为100 mg·L-1的工作液,测定毒力前12 h让甜菜夜蛾幼虫取食经过酶抑制剂浸泡后的甘蓝叶片,对照组取食用0.1% TritonX-100浸泡后的甘蓝叶片,再采用浸叶法进行毒力测定。生物测定48 h后检查试虫死亡率,用软毛笔触碰虫体,不能协调运动的试虫视为死亡。
1.4 解毒酶比活力测定
1.4.1 羧酸酯酶活性测定 参照余慧灵等[13,28]方法,将甜菜夜蛾的冷冻组织与 1 mL磷酸盐缓冲液(0.04 mol·L-1,pH 7.0)冰浴匀浆,并在4℃ 10 000×g条件下离心10 min,取上清液为待测酶液低温储存备用。体系反应体积为3.2 mL,含有50 μL稀释后的酶液,0.45 mL磷酸盐缓冲液与1.8 mL的0.3 mmol·L-1的α-NA溶液(含有0.3 mmol·L-1毒扁豆碱)。30℃温育15 min后加入0.9 mL显色液,对照组在反应结束后再添加酶液,在600 nm比色测定OD值。制作α-萘酚标准曲线与蛋白质含量标准曲线,计算比活力(mmol·min-1·mg-1pro)。
1.4.2 谷胱甘肽 S-转移酶活性测定 参照余慧灵等[13,29]方法,将甜菜夜蛾的冷冻组织与 1 mL含 1.0 mmol·L-1EDTA的磷酸盐缓冲液(0.04 mol·L-1,pH 7.0)冰浴匀浆,并在4℃ 10 000×g条件下离心10 min,取上清液为待测酶液低温储存备用。体系中含有30 μL CDNB(15 mmol·L-1),790 μL磷酸盐缓冲液,30 μL还原型谷胱甘肽(30 mmol·L-1)和50 μL酶液,在340 nm比色记录OD值5 min的变化情况,根据酶液蛋白质含量标准曲线,计算比活力(mmol·min-1·mg-1pro)。
1.4.3 多功能氧化酶 O-脱甲基活力测定 参照余慧灵等[13,30]方法有改动,在将甜菜夜蛾的冷冻组织中加3 mL磷酸盐缓冲液(0.1 mol·L-1,pH 7.8,含0.1 mmol·L-1DTT,0.1 mmol·L-1EDTA,0.1 mmol·L-1PMSF,20%甘油)冰上匀浆,匀浆液于4℃ 10 000×g离心10 min,取上清液为酶液低温储存备用。在酶标板中加入100 μL对硝基苯甲醚(2 mmol·L-1),90 μL酶液再加入10 μL NADPH(0.5 mmol·L-1),30℃温育10 min。在波长405 nm处测定OD值。对照组在温育后加入 NADPH,制作对硝基苯酚制作标准曲线与酶液蛋白质含量标准曲线,计算比活力(nmol·min-1·mg-1pro)。
1.4.4 蛋白质含量的测定 参照 BRADFORD[31]考马斯亮蓝G250法测定,用各处理低温储存的酶液进行蛋白测定。
1.5 两性生命表
采用SE-Lab与SE-Sel品系的F1代构建生命表。参照郝强等[19]方法,在雌虫产卵期的第2天随机收集5个卵块,用0.5%次氯酸钠溶液浸泡30 s后晾干,放入封口袋中,待卵块孵化后挑取 30头初孵幼虫;将150头初孵幼虫用人工饲料饲养,放入指形管(每管放入一头虫),待其化蛹;用0.5%次氯酸钠溶液对蛹行进浸泡消毒,放入六孔细胞培养板中保湿培养待蛹羽化,并将羽化后的成虫编号,根据雌雄个体进行一比一配对,放入圆柱形一次性带盖塑料杯(上口直径×下口直径×高=9.0 cm×5.0 cm×17.0 cm)饲养,提供10%蜂蜜水供成虫补充营养繁殖产卵,如有未配对的成虫,则从初期5个随机收集卵块中成长的成虫中挑选健康的异性成虫进行配对;每天准确记录甜菜夜蛾各个阶段的生长状况、产卵情况,直至成虫死亡。试虫饲养环境条件为温度(25±1)℃、相对湿度(65±10)%和光周期14L﹕10D。
1.6 数据处理
1.6.1 生命表参数 试虫各阶段年龄时间、成虫存活时间及产卵量等原始数据参照年龄-阶段两性生命表理论[9]进行统计,并用程序TWOSEX-MSChart[32]计算相关生命表参数,生命表参数中x表示年龄,j表示试虫发育阶段;年龄-阶段存活率(sxj);种群特定年龄存活率(lx);计算公式:
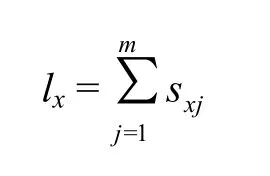
种群特定年龄繁殖力(mx)指整个种群在年龄 x的平均产卵数量,雌虫年龄-阶段繁殖力(fxj),计算公式:

特定年龄-阶段寿命期望值(exj)是指在x年龄j阶段的个体可以继续存活的时间,年龄-阶段生殖值(vxj)就是在x年龄j阶段的个体为种群的增长的贡献值;种群特定年龄存活率(lx)与种群特年龄繁殖力(mx)的乘积为种群特异性年龄繁殖值(lxmx)。内禀增长率(r)指种群在理想状态下的最大种群增长率;周限增长率(λ)指种群在理想状态下,种群内平均每个个体能产生的后代数;净增殖率(R0)指个体的总后代数;平均世代周期(T)指当一个种群达到稳定增长速率时,增加到 R0所需要的时间。计算公式:
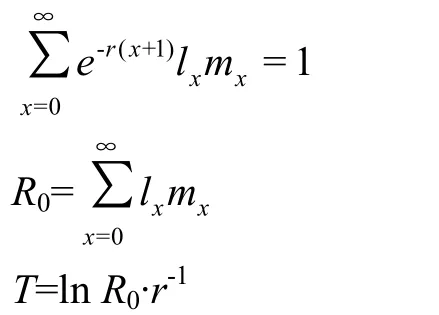
生命表参数数据使用Sigma plot 12.5作图,生命表参数的平均值和标准误用bootstrap技术[33]来估算,用Paired bootstrap test(TWOSEX-MSChart)程序[32]计算甜菜夜蛾发育历期、繁殖值和种群参数间的差异显著性。
1.6.2 生物测定数据分析 采用POLO-Plus10.0软件计算毒力参数。采用SPSS Statistics 19.0软件分析酶活性等的差异显著性。
2 结果
2.1 氯虫苯甲酰胺对甜菜夜蛾幼虫的亚致死剂量
氯虫苯甲酰胺对SE-Lab品系48 h的亚致死剂量LC25为0.017 µg·g-1,对SE-Sel品系48 h亚致死剂量LC25为0.041 µg·g-1。因此,甜菜夜蛾SE-Lab品系经过LC25亚致死剂量连续汰选6代后,其对氯虫苯甲酰胺的敏感度降至汰选前的48.5%(表1)。
2.2 酶抑制剂对甜菜夜蛾幼虫毒力的影响
对SE-Lab与SE-Sel品系分别用酶抑制剂进行处理,SE-Lab品系经过TPP和DEM处理后抗性倍数分别增加1.06、1.47倍,经过PBO处理抗性倍数增加1.58倍。SE-Sel品系在TPP和DEM处理后抗性倍数分别增加1.05、1.51倍,经过PBO处理后增加1.69倍。其中PBO和DEM在SE-Sel品系的增效效果高于SE-Lab品系(表2)。
2.3 氯虫苯甲酰胺亚致死剂量与增效剂对甜菜夜蛾幼虫体内主要代谢解毒酶活性的影响
各处理中肠3种解毒酶活性普遍高于脂肪体中酶

表1 氯虫苯甲酰胺对甜菜夜蛾4龄幼虫的毒力Table 1 Toxicity of chlorantraniliprole to the 4th instar larvae of S. exigua
表2 氯虫苯甲酰胺与氯虫苯甲酰胺+酶抑制剂对甜菜夜蛾4龄幼虫毒力
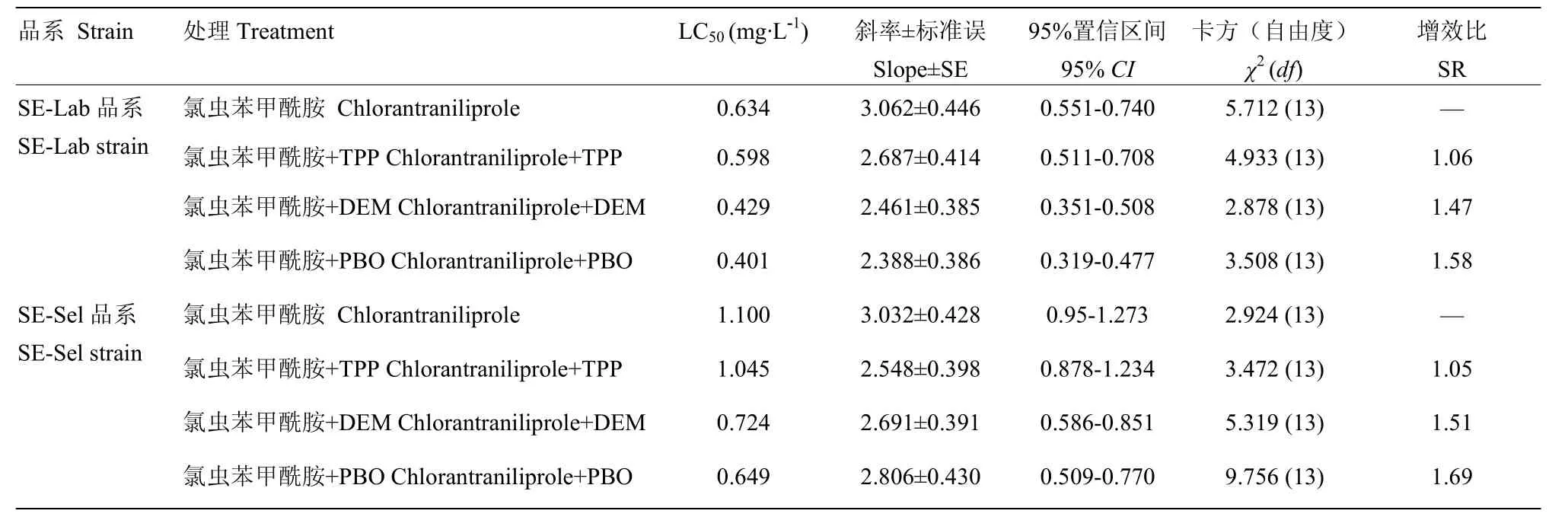
增效比SR (synergism ratio):试虫品系对氯虫苯甲酰胺LC50与在使用增效剂后的氯虫苯甲酰胺LC50的比值LC50of a strain treated with chlorantraniliprole alone divided by LC50of the same strain treated with chlorantraniliprole plus enzyme inhibitors
Table 2 Responses of the 4th instar larvae to chlorantraniliprole or chlorantraniliprole plus enzyme inhibitors in the SE-Lab and SE-Sel strains of S. exigua活性,且SE-Sel品系3个解毒酶活性为SE-lab品系的1.07—2.10倍,其中多功能氧化酶增长倍数最高,中肠和脂肪体分别增长2.07与2.10倍;氯虫苯甲酰胺LD50剂量对SE-Sel品系再次诱导处理48 h后酶活性表明,再次诱导处理的试虫3种解毒酶活性较SE-Sel品系均有有不同程度的增加,尤其以多功能氧化酶增长最明显,在中肠和脂肪体中分别比SE-Lab品系增加了3.44与4.02倍,羧酸酯酶与谷胱甘肽S-转移酶为SE-Lab品系的1.30—2.61倍;而以增效剂+氯虫苯甲酰胺LD50剂量处理SE-Sel品系试虫,解毒酶活结果表明,试虫酶活性较只用药剂的酶活性有显著降低,尤其以PBO处理的多功能氧化酶效果最为明显。在使用酶抑制剂后,3种解毒酶活性均有降低,其中多功能氧化酶活性下降最多,其酶活力值仅为未使用酶抑制剂处理的 42.3%—44.8%(表3)。
表3 各处理的甜菜夜蛾中肠与脂肪体解毒酶比活力
Table 3 The specific activities of detoxification enzymes in midgut and fatbody of S. exigua

表中数据为平均值±标准误,同列数据后含有相同字母表示差异不显著,括号中为每个处理数值与第一行处理数据的比值 Data in the table were represented as mean±SE. The same letter after the data in the same column indicated no significant difference. Data in brackets indicated induced fold compared with data of first row;*酶抑制剂Enzyme inhibitors:使用需要测定的3种解毒酶的专性抑制剂,羧酸酯酶使用TTP,谷胱甘肽S-转移酶使用DEM,多功能氧化酶使用PBO Specific inhibitors of three detoxifying enzymes needed to be measured. Carboxylesterases used TTP, glutathione S-transferases used DEM, multifunctional oxidases used PBO
2.4 氯虫苯甲酰胺亚致死剂量与甜菜夜蛾两性生命表
2.4.1 氯虫苯甲酰胺亚致死剂量对甜菜夜蛾生长发育与繁殖情况的影响 在3龄、4龄与预蛹以外的时期,SE-Lab与SE-Sel品系生长发育历期均有显著差异;SE-Sel品系的成虫前期较SE-Lab品系显著增加,SE-Sel品系的产卵前期(APOP)及总产卵前期(TPOP)较 SE-Lab品系也有所延长;两个品系雄虫的寿命并无显著差异,但雌虫寿命SE-Lab品系显著低于SE-Sel品系,在SE-Sel品系中雄虫比例较SE-Lab品系有所增加;两个品系的平均单雌产卵量有显著差异,SE-Lab品系为995.61粒,SE-Sel品系为743.12粒(表4)。
2.4.2 氯虫苯甲酰胺亚致死剂量对甜菜夜蛾存活率与繁殖力的影响 年龄-阶段特征存活率(sxj)曲线(图1)表示甜菜夜蛾从初产卵活到年龄x和阶段j的可能性,在各个体之间复杂的生长发育阶段形成了大量的时间重叠,自5龄幼虫开始,后期的生长中,SE-Sel品系较 SE-Lab品系存活率有一个明显的下降;计算各品系中由卵完全发育为成虫的概率,SE-Sel品系为51.3%,SE-Lab品系为76.7%。
图2中SE-Sel与SE-Lab品系的种群特定年龄存活率(lx)在生长阶段的前期与后期各有一个较陡的斜线,这两个时期为种群死亡高发期,对应年龄阶段为卵过度到2龄幼虫的时期与成虫后期,其中SE-Sel品系死亡率更高;两个品系的种群特定年龄繁殖力(mx)呈现先增后降的趋势,并在30—38 d达到繁殖高峰,SE-Sel品系的繁殖高峰产卵为70.25粒,SE-Lab品系为88.40粒,雌虫年龄-阶段繁殖力(fxj)在SE-Lab品系呈现先增后降的曲线,但在SE-Sel中在雌虫产卵初期即出现产卵峰,之后产生一定程度波动。
特定年龄-阶段寿命期望值(exy)曲线(图3)表示年龄x阶段j的个体预期能存活的总时间。随着年龄的增长,寿命期望值会随之降低,SE-Lab品系的寿命期望值高于SE-Sel品系。
年龄-阶段生殖值(vxj)曲线(图4) 表示年龄x阶段j的个体对未来种群的贡献,两个品系的雌成虫随着龄期的增加而达到生殖高峰,SE-Lab品系在29 d达到高峰,生殖力为634.6粒,SE-Sel品系在产卵早期即达到最高峰,在25 d时达到700.1粒。
2.4.3 氯虫苯甲酰胺亚致死剂量对甜菜夜蛾生命表种群参数的影响 SE-Sel品系的内禀增长率(r)、周限增长率(λ)、净增殖率(R0)分别为0.18、1.20与358.42 d-1,SE-Lab品系分别为0.16、1.17与203.12 d-1,且SE-Sel品系显著低于SE-Lab品系;SE-Sel品系平均世代周期(T)与Se-Lab品系差异不显著(表5)。
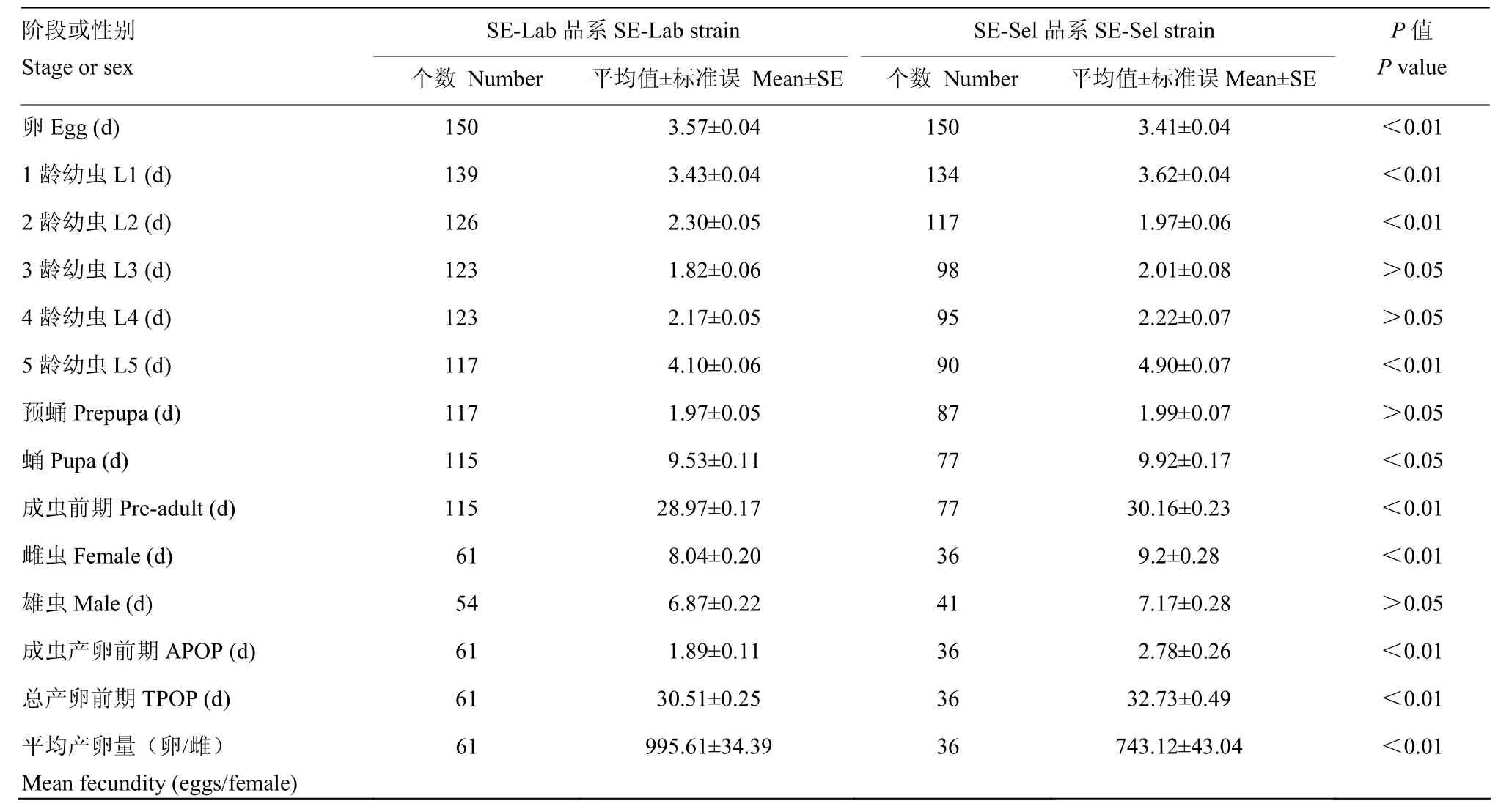
表4 甜菜夜蛾在氯虫苯甲酰胺亚致死剂量作用下的基本生活史参数Table 4 Parameters of life history of sublethal of chlorantraniliprole on developmental duration of S. exigua

图1 氯虫苯甲酰胺亚致死剂量对甜菜夜蛾年龄-阶段特征存活率(sxj)的影响Fig. 1 The effects of chlorantraniliprole sublethal dosage on age-stage specific survival rate (sxj) of S. exigua
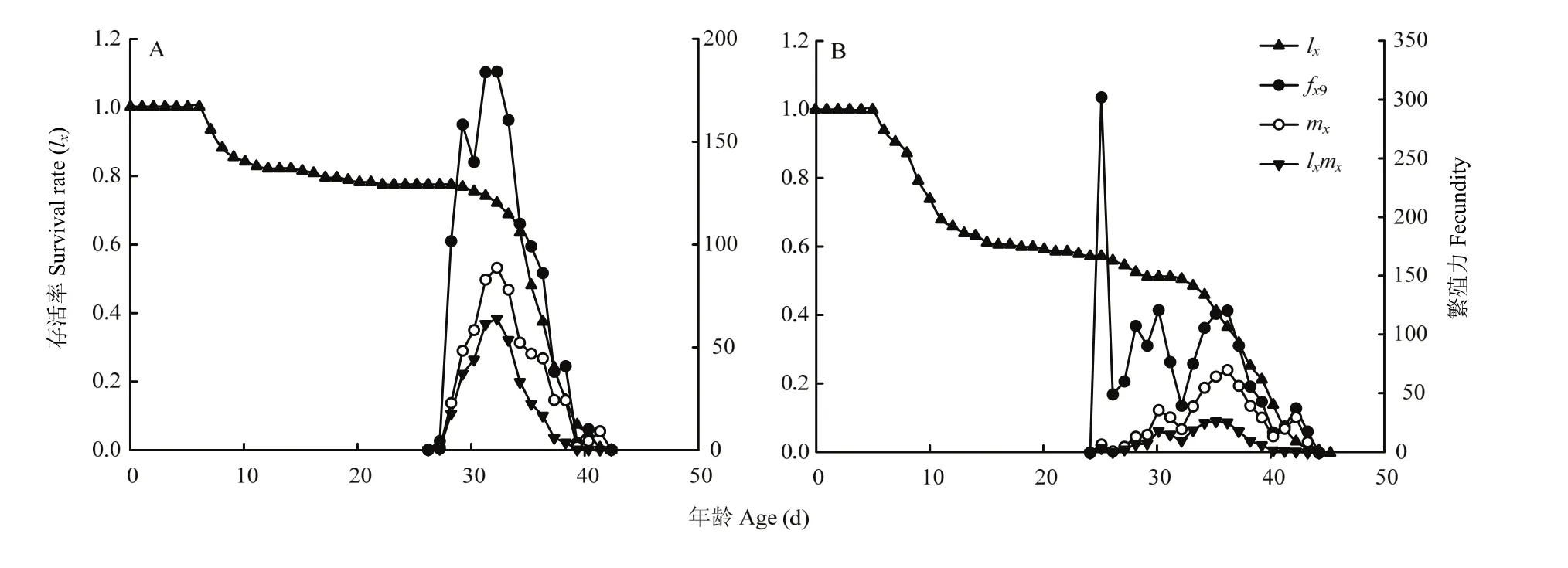
图 2 氯虫苯甲酰胺亚致死剂量对甜菜夜蛾种群特定年龄存活率(lx)、雌虫年特定年龄繁殖力(fx9)、种群特定年龄繁殖力(mx)和种群特定年龄繁殖值(lxmx)的影响Fig. 2 The effects of chlorantraniliprole sublethal dosage on age-specific survival rate (lx), female age-specific fecundity (fx9), age-specific fecundity of total population (mx), and age-specific maternity (lxmx) of S. exigua

图3 氯虫苯甲酰胺亚致死剂量对甜菜夜蛾年龄-阶段特征寿命期望值(exj)的影响Fig. 3 The effects of chlorantraniliprole sublethal dosage on age-stage specific life expectancies (exj) of S. exigua

表5 氯虫苯甲酰胺亚致死剂量对甜菜夜蛾生命表参数的影响Table 5 The effects of sublethal dosage of chlorantraniliprole on population parameters of S. exigua
3 讨论
杀虫剂亚致死剂量处理对昆虫生长发育、繁殖力及抗性等有相应程度的影响[34]。刑静等[35]报道,氯虫苯甲酰胺亚致死剂量处理小菜蛾3龄幼虫24、48和72 h,试虫体内的细胞色素P450 O-脱乙基酶(ECOD)和谷胱甘肽S-转移酶(GSTs)酶活性均被抑制;在使用PBO、DEM、TPP酶抑制剂后,SE-Lab品系和抗性品系的小菜蛾的抗性均有相应的增加,其中 DEM增效效果最佳,对抗性品系的小菜蛾使用氯虫苯甲酰胺短期诱导后,其GSTs酶活性也显著增加[36];据余慧灵等[13]报道,以LC10和LC25剂量处理甜菜夜蛾3龄幼虫24 h,3种解毒酶活性均有相应的增加。本试验中,SE-Sel品系的GSTs与MFOs酶活性较SE-Lab有明显的增加,而在用亚致死剂量氯虫苯甲酰胺诱导SE-Sel品系后3种解毒酶活性均有一定程度的上升,其中MFOs活性增长倍数最高,根据昆虫抗药性机理,多功能氧化酶可能与甜菜夜蛾对氯虫苯甲酰胺产生抗性有关,并与表达相关解毒酶的基因相关[37-38]。
两性生命表中研究雄虫的参数,在传统生命表中是相对缺乏的,ZHANG等[39]用氯虫苯甲酰胺亚致死剂量处理棉铃虫后,F1代雄虫比例增加;DELPUECH等[40]研究表明,在亚致死剂量药剂压力下,昆虫间的信息素传递受到影响,使雌成虫生殖力降低使后代雄虫数量的增加,从而影响种群的性别分布。由于卵子受精是由中枢神经系统控制的,而杀虫剂通过影响神经传导干扰卵子受精致使受精卵的减少,来控制昆虫性别比例变化[41]。本研究中,SE-Sel品系雄成虫与雌成虫的性别比例较 SE-Lab品系有所增加,与前人研究结果变化趋势一致。宋月芹等[42]研究表明,用氯虫苯甲酰胺LC10剂量处理试虫后,亚洲玉米螟(Ostrinia furnacalis)成虫寿命变长且雌成虫寿命明显延长;杨洪等[43]用氯虫苯甲酰胺 LC25剂量处理白背飞虱(Sogatella furcifera)后,雌成虫寿命都有一定的缩短;而LAI等[44]采用氯虫苯甲酰胺亚致死剂量处理甜菜夜蛾幼虫后,则发现成虫寿命没有显著变化。本研究表明,SE-Sel品系雌成虫寿命相对于SE-Lab品系雌成虫有一定程度的增加。因此,不同昆虫在不同环境下,经不同药剂处理可能对昆虫雌成虫寿命有不同影响。

图4 氯虫苯甲酰胺亚致死剂量对甜菜夜蛾的年龄-阶段特征生殖能力(vxj)的影响Fig. 4 The effects of chlorantraniliprole sublethal dosage on age-stage specific reproductive values (vxj) of S. exigua
杀虫剂亚致死剂量对昆虫所产生的效应与施用杀虫剂种类和浓度水平、受药昆虫的种类和生理状况有关系。GUO等[45]报道,小菜蛾取食含LC25剂量氯虫苯甲酰胺的甘蓝叶片后,世代周期增长,净增殖率明显降低,此现象有跨代效应,在F1代中仍然出现,统计试虫死亡率发现经药剂处理试虫的 F1代较对照组显著增加;HAN等[46]用氯虫苯甲酰胺LC10、LC25剂量处理小菜蛾后,发现试虫发育历期明显增长,雌成虫产卵量也有相应程度的降低;陈琼等[47]用氯虫苯甲酰胺LC25处理甜菜夜蛾后也得到相似结果。但是,也有杀虫剂在诱导昆虫亲代后出现猖獗现象并促进昆虫繁殖率的报道,如绿盲蝽(Apolygus lucorum)[48]、大螟(Sesamia inferens)[49]和小菜蛾[50]等。本研究中,SE-Sel品系经过连续6代的药剂亚致死剂量处理,较传统通过药剂处理1代的亚致死效应研究,更类似于田间环境下杀虫剂对连续多代害虫的影响,SE-Sel品系在各年龄阶段的存活率与雌成虫繁殖力较 SE-Lab品系有明显的下降的现象,该现象可能与氯虫苯甲酰胺处理的跨代效应有关。由于连续的药剂诱导,试虫对杀虫剂耐受能力增强,但甜菜夜蛾的幼虫存活率、繁殖力、内禀增长率、周限增长率、净增殖率等种群参数均有下降。YIN等[51]使用多抗菌素亚致死剂量连续5代诱导小菜蛾,小菜蛾的种群增长受到抑制,其繁殖力、内禀增长率、周限增长率、净增殖率等参数均有一定程度下降;刘泽文等[52]研究发现,田间采集的抗吡虫啉褐飞虱(Nilaparvata lugens)种群与敏感品系相比,相对适合度有明显下降,田间种群表现出繁殖不利现象;陈朗杰等[53]通过对抗性品系与敏感品系的橘小实蝇(Bactrocera dorsalis)的种群生物学参数进行比较,发现高抗性品系的繁殖力和种群世代增长量受到抑制,中抗品系尤为明显。抗药性增加了害虫抗性个体在药剂选择压力下的存活率,但由于药剂的持续压力使害虫的生存繁殖力下降,以至于抗性种群与敏感种群相比并没有明显生存竞争优势。氯虫苯甲酰胺亚致死剂量的连续处理虽然对甜菜夜蛾的种群增长有抑制或减缓的作用,但也会使其逐渐的形成抗药性。
本研究从解毒酶及生命表参数两方面分析氯虫苯甲酰胺亚致死剂量对甜菜夜蛾的影响,可为进一步从分子角度研究与抗药性相关的解毒代谢酶基因提供依据;两性生命表研究可更直观地了解连续亚致死剂量的氯虫苯甲酰胺对甜菜夜蛾各龄期的生命参数影响,是对传统生命表研究的重要补充,为田间害虫的药剂防控提供理论依据。
4 结论
多功能氧化酶可能为甜菜夜蛾对氯虫苯甲酰胺解毒代谢过程中的主要解毒酶,在其后续抗性形成中起主要作用;甜菜夜蛾在氯虫苯甲酰胺亚致死剂量作用下,世代周期延长,繁殖力降低,种群增长减缓,氯虫苯甲酰胺亚致死剂量对甜菜夜蛾有持续控制作用。
[1] JAKUBOWSKA A K, LYNN D E, HERRERO S, VLAK J M, VAN OERS M M. Host-range expansion of Spodoptera exigua multiple nucleopoly hedrovirus to Agrotis segetum larvae when the midgut is bypassed. Journal of General Virology, 2010, 91(4): 898-906.
[2] WU G, GUO J Y, WAN F H, XIAO N W. Responses of three successive generations of beet armyworm, Spodoptera exigua, fed exclusively on different levels of gossypol in cotton leaves. Journal of Insect Science, 2010, 10: Article 165.
[3] LI Y X, MAO M Z, LI Y M, XIONG L X, LI Z M, XU J Y. Modulations of high-voltage activated Ca2+channels in the central neurones of Spodoptera exigua by chlorantraniliprole. Physiological Entomology, 2011, 36(3): 230-234.
[4] TEIXEIRA L A, ANDALORO J T. Diamide insecticides: Global efforts to address insect resistance stewardship challenges. Pesticide Biochemistry and Physiology, 2013, 106(3): 76-78.
[5] SAIL A A, BRUNNER J F. Assessment of resistance risk in obliquebanded leafroller (Lepidoptera: Tortricidae) to the reduced-risk insecticides chlorantraniliprole and spinetoram. Journal of Economic Entomology, 2010, 103(4): 1378-1385.
[6] LAI T C, LI J, SU J Y. Monitoring of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) resistance to chlorantraniliprole in China. Pesticide Biochemistry and Physiology, 2011, 101(3): 198-205.
[7] RAKOTONDRAVELO M L, ANDERSON T D, CHARLTON R E, ZHU K Y. Sublethal effects of three pesticides on activities of selected target and detoxification enzymes in the aquatic midge, Chironomus tentans (Diptera: Chironomidae). Archives of Environmental Contamination and Toxicology, 2006, 51(3): 360-366.
[8] VOJOUDI S, SABER M, HEJAZI M J,TALAEI-HASSANLOUI R. Toxicity of chlorpyrifos, spinosad and abamectin on cotton bollworm, Helicoverpa armigera and their sublethal effects on fecundity and longevity. Bulletin of Insectology, 2011, 64(2): 189-193.
[9] CHI H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environmental Entomology, 1988, 17(1): 26-34.
[10] CHI H, SU H Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environmental Entomology, 2006, 35(1): 10-21.
[11] HUANG Y B, CHI H. The age-stage, two-sex life table with an offspring sex ratio dependent on female age. Journal of Agricultural and Forest, 2011, 60(4): 337-345.
[12] 刘喃喃, 朱芳, 徐强, PRIDGEON J W, 高希武. 昆虫抗药性机理:行为和生理改变及解毒代谢增强. 昆虫学报, 2006, 49(4): 671-679.
LIU N N, ZHU F, XU Q, PRIDGEON J W, GAO X W. Behavioral change, physiological modification, and metabolic detoxification: mechanisms of insecticide resistance. Acta Entomologica Sinica, 2006, 49(4): 671-679. (in Chinese)
[13] 余慧灵, 向兴, 袁贵鑫, 陈羿渠, 王学贵. 溴氰虫酰胺亚致死剂量对甜菜夜蛾生长发育及体内解毒酶活性的影响. 昆虫学报, 2015, 58(6): 634-641.
YU H L, XIANG X, YUAN G X, CHEN Y Q, WANG X G. Effects of sublethal doses of cyantraniliprole on the growth and development and the activities of detoxifying enzymes in Spodoptera exigua (Lepidoptera: Noctuidae). Acta Entomologica Sinica, 2015, 58(6): 634-641. (in Chinese)
[14] WANG W, MO J C, CHENG J A, ZHUANG P J, TANG Z H. Selection and characterization of spinosad resistance in Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Pesticide Biochemistry and Physiology, 2006, 84(3): 180-187.
[15] TIAN X R, SUN X X, SU J Y. Biochemical mechanisms for metaflumizone resistance in beet armyworm, Spodoptera exigua. Pesticide Biochemistry and Physiology, 2014, 113(1): 8-14.
[16] 贾变桃, 焦鹏, 杨素梅. 虱螨脲亚致死浓度对小菜蛾保护酶系和解毒酶系活力的影响. 植物保护学报, 2016, 43(2): 293-299.
JIA B T, JIAO P, YANG S M. Effects of sublethal concentrations of lufenuron on endogenous protective and detoxifying enzymes in the diamondback moth, Plutella xylostella (L.). Journal of Plant Protection, 2016, 43(2): 293-299. (in Chinese)
[17] 欧善生, 梁沛, 宋敦伦, 史雪岩, 高希武. 氯虫苯甲酰胺亚致死剂量对棉铃虫生长发育和解毒酶活性的影响. 植物保护, 2012, 38(4): 1-8.
OU S S, LIANG P, SONG D L, SHI X Y, GAO X W. Effects of sublethal dosage of chlorantraniliprole on development and detoxifying enzymes activity of Helicoverpa armigera. Plant Protection, 2012, 38(4): 1-8. (in Chinese)
[18] 黄诚华, 姚洪渭, 叶恭银, 程家安. 氟虫腈亚致死剂量处理对二化螟和大螟幼虫体内解毒酶系活力的影响. 中国水稻科学, 2006, 20(4): 447-450.
HUANG C H, YAO H W, YE G Y, CHENG J A. Effects of sublethal dose of fipronil on detoxifying enzymes in the larvae of Chilo suppressalis and Sesamia inferens. Chinese Journal of Rice Science, 2006, 20(4): 447-450. (in Chinese)
[19] 郝强, 黄倩, 梁炜博, 贡常委, 王学贵. 不同温度下斜纹夜蛾的两性生命表. 昆虫学报, 2016, 59(6): 654-662.
HAO Q, HUANG Q, LIANG W B, GONG C W, WANG X G. Age-stage two-sex life tables of Spodoptera litura (Lepidoptera: Noctuidae) at different temperatures. Acta Entomologica Sinica, 2016, 59(6): 654-662. (in Chinese)
[20] TUAN S J, LI N J, YEH C C, TANG L C, CHI H. Effects of green manure cover crops on Spodoptera litura (Lepidoptera: Noctuidae) populations. Journal of Economic Entomology, 2014, 107(3): 897-905.
[21] JHA R K, TUAN S J, CHI H, TANG L C. Life table and consumption capacity of corn earworm, Helicoverpa armigera, fed asparagus, Asparagus officinalis. Journal of Insect Science, 2014, 14: Article 34.
[22] 王海鸿, 薛瑶, 雷仲仁. 恒温和波动温度下西花蓟马的实验种群生命表. 中国农业科学, 2014, 47(1): 61-68.
WANG H H, XUE Y, LEI Z R. Life tables for experimental populations of Frankliniella occidentalis (Thysanoptera: Thripidae) under constant and fluctuating temperature. Scientia Agricultura Sinica, 2014, 47(1): 61-68. (in Chinese)
[23] ESMAEILY S, SAMIH M A, ZARABI M, JAFARBEIGI F. Sublethal effects of some synthetic and botanical insecticides on Bemisia tabaci (Hemiptera: Aleyrodidae). Journal of Plant Protection Research, 2014, 54(2): 171-178.
[24] JAFARBEIGI F, SAMIH M A, ZARABI M, ESMAEILY S. Age stage two-sex life table reveals sublethal effects of some herbal and chemical insecticides on adults of Bemisia tabaci (Hem.: Aleyrodidae). Psyche A Journal of Entomology, 2014, 2014(3): 1-9.
[25] RAHMANI S, BANDANI A R. Sublethal concentrations of thiamethoxam adversely affect life table parameters of the aphid predator, Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae). Crop Protection, 2013, 54(12): 168-175.
[26] SCHNEIDER M I, SANCHEZ N, PINEDA S, CHI H, RONCO A. Impact of glyphosate on the development, fertility and demography of Chrysoperla externa (Neuroptera: Chrysopidae): Ecological approach. Chemosphere, 2009, 76(10): 1451-1455.
[27] HUANG Y B, CHI H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Science, 2012, 19(2): 263-273.
[28] ASPEREN K V. A study of housefly esterases by means of a sensitive colorimetric method. Journal of Insect Physiology, 1962, 8(4): 401-416.
[29] HABIG W H, JAKOBY W B. Assays for differentiation of glutathione S-transferases. Methods in Enzymology, 1981, 77: 398-405.
[30] ROSE R L, BARBHAIYA L, ROE R M, ROCK G C, HODGSON E. Cytochrome P450-associated insecticide resistance and the development of biochemical diagnostic assays in Heliothis virescens. Pesticide Biochemistry and Physiology, 1995, 51(3): 178-191.
[31] BRADFORD M M A. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 1976, 25(1): 248-256.
[32] CHI H. TWOSEX-MSChart: A computer program for the age stage, two-sex life table analysis. National Chung Hsing University, Taichung, Taiwan. 2016. http://140.120.197.173/Ecology/.
[33] HUANG Y B, CHI H. Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): with an invalidation of the jackknife technique. Journal of Applied Entomology, 2013, 137(5): 327-339.
[34] DESNEUX N, DECOURTYE A, DELPUECH J M. The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology, 2007, 52(1): 81-106.
[35] 邢静, 梁沛, 高希武. 亚致死浓度氯虫苯甲酰胺对小菜蛾药剂敏感度和解毒酶活性的影响. 农药学学报, 2011, 13(5): 464-470.
XING J, LIANG P, GAO X W. Effects of sublethal concentrations ofchlorantraniliprole on insecticide susceptibility and detoxifying enzyme activity in Plutella xylostella. Chinese Journal of Pesticide Science, 2011, 13(5): 464-470. (in Chinese)
[36] HU Z D, FENG X, LIN Q S, CHEN H Y, LI Z Y, YIN F, LIANG P, GAO X W. Biochemical mechanism of chlorantraniliprole resistance in the diamondback moth, Plutella xylostella, Linnaeus. Journal of Integrative Agriculture, 2014, 13(11): 2452-2459.
[37] ZHONG D, CHANG X, ZHOU G, HE Z, FU F, YAN Z, ZHU G, XU T, BONIZZONI M, WANG MH, CUI L, ZHENG B, CHEN B, YAN G. Relationship between knockdown resistance, metabolic detoxification and organismal resistance to pyrethroids in Anopheles sinensis. PLoS ONE, 2013, 8(2): e55475.
[38] LI X C, SCHULER M A, BERENBAUM M R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annual Review of Entomology, 2007, 52(1): 231-253.
[39] ZHANG R M, DONG J F, CHEN J H, QING E J, CUI J J. The sublethal effects of chlorantraniliprole on Helicoverpa armigera (Lepidoptera: Noctuidae). Journal of Integrative Agriculture, 2013, 12(3): 457-466.
[40] DELPUECH J M, GAREAU E, TERRIER O, FOUILLET P. Sublethal effects of the insecticide chlorpyrifos on the sex pheromonal communication of Trichogramma brassicae. Chemosphere, 1998, 36(8): 1775-1785.
[41] DELPUECH J M, MEYET J. Reduction in the sex ratio of the progeny of a parasitoid wasp (Trichogramma brassicae) surviving the insecticide chlorpyrifos. Archives of Environmental Contamination and Toxicology, 2003, 45(2): 203-208.
[42] 宋月芹, 董钧锋, 孙会忠. 亚致死浓度氯虫苯甲酰胺可降低亚洲玉米螟的种群增长. 昆虫学报, 2013, 56(4): 446-451.
SONG Y Q, DONG J F, SUN H Z. Chlorantraniliprole at sublethal concentrations may reduce the population growth of the Asian corn borer, Ostrinia furnacalis (Lepidoptera: Pyralidae). Acta Entomologica Sinica, 2013, 56(4): 446-451. (in Chinese)
[43] 杨洪, 王召, 金道超. 氯虫苯甲酰胺对白背飞虱实验种群的亚致死效应. 昆虫学报, 2012, 55(10): 1161-1167.
YANG H, WANG Z, JIN D C. Sublethal effects of chlorantraniliprole on the experimental populations of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Acta Entomologica Sinica, 2012, 55(10): 1161-1167. (in Chinese)
[44] LAI T C, SU J A. Effects of chlorantraniliprole on development and reproduction of beet armyworm, spodoptera exigua, (Hübner). Journal of Pest Science, 2011, 84(3): 381-386.
[45] GUO L, DESNEUX N, SONODA S, LIANG P, HAN P, GAO X W. Sublethal and transgenerational effects of chlorantraniliprole on biological traits of the diamondback moth, Plutella xylostella L. Crop Protection, 2013, 48(2): 29-34.
[46] HAN W S, ZHANG S F, SHEN F Y, LIU M, REN C C, GAO X W. Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae). Pest Management Science, 2012, 68(8): 1184-1190.
[47] 陈琼, 黄水金, 秦文婧. 氯虫苯甲酰胺对甜菜夜蛾的亚致死效应研究. 江西农业大学学报, 2011, 33(4): 690-695.
CHEN Q, HUANG S J, QIN W J. Sublethal effects of chlorantraniliprole in Spodoptera exigua. Acta Agriculturae Universitis Jiangxiensis, 2011, 33(4): 690-695. (in Chinese)
[48] TAN Y, BIONDI A, DESNEUX N, GAO X W. Assessment of physiological sublethal effects of imidacloprid on the mirid bug Apolygus lucorum (Meyer-Dür). Ecotoxicology, 2012, 21(7): 1989-1997.
[49] 杨国庆, 李丽, 戈林泉, 吴进才. 三唑磷和毒死蜱亚致死剂量对大螟种群增长和卵黄蛋白含量的影响. 应用昆虫学报, 2014, 51(6): 1582-1588.
YANG G Q, LI L, GE L Q, WU J C. Effects of sublethal doses of triazophos and chlorpyrifos on the population growth and yolk protein content of Sesamia inferens (Walker). Chinese Journal of Applied Entomology, 2014, 51(6): 1582-1588. (in Chinese)
[50] MAHMOUDVAND M, ABBASIPOUR H, GARJAN A S, BANDANI A R. Sublethal effects of hexaflumuron on development and reproduction of the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Insect Science, 2011, 18(6): 689-696.
[51] YIN X H, WU Q J, LI X F, ZHANG Y J, XU B Y. Demographic changes in multigeneration Plutella xylostella (Lepidoptera: Plutellidae) after exposure to sublethal concentrations of spinosad. Journal of Economic Entomology, 2009, 102(1): 357-365.
[52] 刘泽文, 刘成君, 张洪伟, 韩召军. 褐飞虱抗吡虫啉品系生物适合度研究. 应用昆虫学报, 2003, 40(5): 419-422.
LIU Z W, LIU C J, ZHANG H W, HAN Z J. Relative biological fitness of imidacloprid resistant strains of Nilaparvata lugens. Chinese Journal of Applied Entomology, 2003, 40(5): 419-422. (in Chinese)
[53] 陈朗杰, 刘昕, 吴善俊, 朱弋凡, 曾玲, 陆永跃. 桔小实蝇抗敌百虫品系的实验种群生物学比较研究. 昆虫学报, 2015, 58(8): 864-871.
CHEN L J, LIU X, WU S J, ZHU Y F, ZENG L, LU Y Y. A comparative study of the population biology of trichlorfon- resistant strains of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritdae). Acta Entomologica Sinica, 2015, 58(8): 864-871. (in Chinese)
(责任编辑 岳梅)
Effects of Sublethal Doses of Chlorantraniliprole on the Detoxification Enzymes Activities and the Growth and Reproduction of Spodoptera exigua
CHEN YiQu, XIANG Xin, GONG ChangWei, WANG XueGui
(Biorational Pesticide Research Laboratory, College of Agronomy, Sichuan Agricultural University, Chengdu 611130)
Spodoptera exigua; chlorantraniliprole; sublethal doses; synergists; MFOs; age-stage two-sex life tables
2016-12-12;接受日期:2017-02-27
国家公益性行业(农业)科研专项(201203038)
联系方式:陈羿渠,Tel:028-86290977;E-mail:chenyiqu123@163.com。通信作者王学贵,Tel:028-86290977;E-mail:wangxuegui@sicau.edu.cn
Abstract:【Objective】 Spodoptera exigua is a polyphagous pest and shows different characteristics of growth and development under the selective pressure of different environments, drugs and so on. Chlorantraniliprole, a novel insecticide that acts on ryanodine receptors, has highly activity to those insects in Lepidopteran. The objective of this study is to explore the effects of S. exigua larvae treated by the sublethal doses of chlorantraniliprole on the toxicities, the activities of three main detoxifying enzymes, including carboxylesterase (CarE), glutathione S-transferase (GSTs), and mixed-function oxidase (MFOs), and population breeding.【Method】 The toxicities of chlorantraniliprole on SE-Lab and SE-Sel strains were detected by incorporation bioassay and the SE-Sel strain was achieved with continuous selecting with LC25sublethal doses for six generations from SE-Lab strain, then the synergistic effects of enzyme inhibitors (TPP, DEM, PBO) with chlorantraniliprole on the SE-Lab and SE-Sel strains were assayed using the dip-leaf method. The toxicities of chlorantraniliprole on the insects which were fed the leaves soaked with the solution of enzyme inhibitor or 0.1% TritonX-100 as blank control before 12 h were assayed by the dip-leaf method. The midgut and fatbody of tested insects were dissected on the ice and the effects of the sublethal doses of chlorantraniliprole and enzyme inhibitors on three metabolic detoxification enzyme activities were analyzed by the determination of detoxification enzyme activities. According to the age-stage two-sex life tables theory, the growth, mortality, fecundity data of the tested insects were also recorded to analyze the differences of the age-stage two-sex life table parameters between the strains of SE-Lab and SE-Sel. 【Result】 The synergistic effect of the PBO was the strongest among the three enzyme inhibitors and the synergic ratios on the strains of SE-Sel and SE-Lab reached 1.58- and 1.69-fold, respectively. The activities of the three detoxification enzymes, which were induced by the continuous selection of the sublethal doses of chlorantraniliprole, were promoted and the MFOs activities were the most significant, which of the SE-Sel strain in the midgut and fatbody were enhanced by 2.07- and 2.10-fold, meanwhile, the MFOs activities of the insects of SE-Sel induced by the sublethal dose of chlorantraniliprole again were also promoted by 4.02- and 3.44-fold in the midgut and fatbody compared to those in SE-Lab strain, respectively. The enzyme activities of three detoxifying enzymes were decreased when the tested insects were treated with enzyme inhibitors and the descend range of MFOs activity among the three enzymes was maximum, which was only 42.3%-44.8% compared to the treatment not treated with the enzyme inhibitor. The adult preoviposition period and total preoviposition period of F1generation of SE-Sel strains became shorter and spawning quantity of became higher compared to those in F1generation of SE-Lab. SE-Sel strains had the longest mean generation time and least amount of eggs. The intrinsic rate of increase (r), finite rate of increase (λ) and net reproductive rate (R0) of SE-Sel strain were significantly lower than those in SE-Lab strain. The r, λ and R0values of SE-Lab and SE-Sel strains were 0.18 and 0.16 d-1, 1.20 and 1.17 d-1, 358.42 and 203.12 d-1, respectively. Even though the mean generation times of SE-Sel strain was longer than SE-Lab strain, there was no significant difference between the two strains.【Conclusion】MFOs may be the major detoxication enzyme on the metabolic detoxification of chlorantraniliprole in the S. exigua and involved in the formation of resistance. A longer generation period, lower fecundity and slower population growth of S. exigua were demonstrated when it was treated by a continuous selection of lethal dose of chlorantraniliprole. The sublethal doses of chlorantraniliprole have a continuous control effect on S. exigua.

