苹果炭疽叶枯病菌GcAP1复合体β亚基基因的克隆及功能分析
张俊祥,冀志蕊,王娜,徐成楠,迟福梅,周宗山
(中国农业科学院果树研究所,辽宁兴城 125100)
苹果炭疽叶枯病菌GcAP1复合体β亚基基因的克隆及功能分析
张俊祥,冀志蕊,王娜,徐成楠,迟福梅,周宗山
(中国农业科学院果树研究所,辽宁兴城 125100)
【目的】明确衔接蛋白(adaptor protein)GcAP1复合体β亚基在苹果炭疽叶枯病菌(Glomerella cingulata)生长发育和致病过程中的功能,检测GcAP1β在该菌中的时空表达模式,并揭示其是否调控多聚半乳糖醛酸内切酶(endopolygalacturonase)基因CgPG1和CgPG2、果胶裂解酶(pectin lyase)基因pnl-1和pnl-2以及果胶酸酯裂解酶(pectate lyase)基因pelA和pelB的表达,为深入开展苹果炭疽叶枯病菌衔接蛋白在致病信号传导途径中的分子机制研究打下基础。【方法】通过构建GcAP1β基因敲除载体和GcAP1β-gfp融合表达载体,利用农杆菌介导的遗传转化技术(ATMT)获得Δgcap1β突变体和GcAP1β恢复菌株Δgcap1β-GcAP1β,并由RT-PCR和Southern杂交分析进行鉴定。以野生型菌株W16为对照,对Δgcap1β突变体和GcAP1β恢复菌株Δgcap1β-GcAP1β的生长速度、产孢能力、分生孢子萌发率及附着胞形成率和致病性进行测定。利用生物信息学软件ProtComp 9.0和TMHMM对GcAP1β蛋白进行结构分析,并结合GcAP1β-GFP信号观测,进行GcAP1β的亚细胞定位。利用qRT-PCR技术,检测GcAP1β在菌丝、分生孢子、芽管、附着胞和侵染阶段的表达量,并检测CgPG1、 CgPG2、pnl-1、pnl-2、pelA和pelB在野生型菌株和Δgcap1β突变体中的表达量。【结果】GcAP1β基因全长2 321 bp,含有3个内含子,编码720个氨基酸。与野生型菌株W16相比,Δgcap1β突变体菌落成褶皱状,菌丝生长速度明显减慢,而分生孢子产量、分生孢子萌发率、附着胞形成率无显著差异。Δgcap1β致病力明显降低,仅在苹果叶片上引起极小的点状斑。GcAP1β基因恢复菌株Δgcap1β-GcAP1β完全修复了因GcAP1β基因缺失造成的表型缺陷。荧光检测显示,融合蛋白GcAP1β-GFP分布于细胞质中。qRT-PCR检测结果表明,GcAP1β在苹果炭疽叶枯病菌各个发育阶段都有表达,且在侵染后表达量相对最高。GcAP1β的缺失导致CgPG1表达量降低至20.3%,CgPG2表达量降低至16.5%,pnl-1表达量降低至8.2%,pnl-2表达量降低至14.4%,pelA表达量降低至4.4%,pelB表达量降至0.8%。【结论】衔接蛋白GcAP1复合体分布于细胞质中,是苹果炭疽叶枯病菌生长发育所需要的;GcAP1调控CgPG1、CgPG2、pnl-1、pnl-2、pelA和pelB的表达,是苹果炭疽叶枯病菌一个重要的毒力因子。
衔接蛋白;果胶酶;苹果;炭疽菌;致病力
0 引言
【研究意义】衔接蛋白(adaptor protein,AP)在哺乳动物细胞内信号的传递、蛋白质的分拣及向囊泡转运过程中发挥着重要作用[1],但关于 AP蛋白在真菌中的功能鲜有报道。以GcAP1复合体β亚基基因为出发点,揭示其在苹果炭疽叶枯病菌生长发育和致病过程中的功能,可为植物病害防治的新途径、新方法提供线索,并对植物病原真菌致病机理研究具有重要意义。【前人研究进展】近几年,苹果一种重要的新病害——苹果炭疽叶枯病(Glomerella leaf spot of apple),在中国苹果主产区连年大发生,给苹果产业带来巨额损失[2]。现已发现,围小丛壳菌(Glomerella cingulata)(无性态:胶胞炭疽菌 Colletotrichum gloeosporioides)[3-5]、尖孢炭疽菌(C. acutatum)[6]、喀斯特炭疽菌(C. karstii)[7]、果生刺盘孢(C. fructicola)和隐秘刺盘孢(C. aenigma)[8]均可引起苹果炭疽叶枯病。炭疽菌通过分生孢子萌发,产生芽管和附着胞,附着胞下形成的侵染钉可直接侵入寄主[4]。像其他植物病原真菌一样,炭疽菌(Colletotrichum)侵染过程受cAMP和MAPK路径调控[9]。胶胞炭疽菌通过分泌果胶酶,例如聚半乳糖醛酸酶(polygalacturonase,PG)、果胶裂解酶(pectin lyase,PNL)和果胶酸酯裂解酶(pectate lyase,PEL),克服寄主细胞壁中的果胶,使其成功在寄主体内定殖。研究表明,PG1、 PG2、pnl-1、pnl-2、pelA和pelB单个基因突变或双基因突变均明显地降低病原菌的致病力[10-14]。最近的研究表明,在碱性条件下,转录因子pacC调控pelB的表达[15],但关于果胶酶基因的调控机制尚需进一步研究。AP蛋白以复合体形式存在于生物体中,具有重要的功能[16-17]。在哺乳动物中,现已发现5种类型的AP复合体,即AP-1、AP-2、AP-3、AP-4和AP-5[18-19]。AP蛋白由4个不同的亚基构成(δ、β、μ和σ),2个大亚基(δ和β)具有识别和结合网格蛋白的功能,1个中亚基(μ)负责蛋白的分拣和跨膜装配,小亚基(σ)具有稳定复合体的作用[18,20-21]。目前,胶胞炭疽菌菌株23的基因组测序已完成,其Contig序列公布在“Comparative Fungal Genomics Platform”。此外,博德研究所(http://www.broadinstitute.org)还公布了玉米炭疽菌(C. graminicola)和希金斯刺盘孢(C. higginsiamim)全基因序列。这些基因组信息为炭疽菌的致病机理研究创造了条件。【本研究切入点】为开展苹果炭疽叶枯病菌致病机制研究,笔者课题组之前利用农杆菌介导的遗传转化(ATMT)方法,构建了苹果炭疽叶枯病菌强致病力菌株W16的T-DNA插入突变体库[5]。在对一些T-DNA插入突变体进行致病性测验中,发现一株突变体A7346对苹果叶片及果实致病力明显降低。进一步揭示突变体A7346致病力降低的分子机制,将是研究苹果炭疽叶枯病菌致病机制的一个很好契机。【拟解决的关键问题】阐明 GcAP1复合体 β亚基在苹果炭疽叶枯病菌致病过程中的功能,明确GcAP1复合体是否调控CgPG1、CgPG2、pnl-1、pnl-2、pelA和 pelB的表达,为深入开展 AP蛋白在苹果炭疽叶枯病菌致病信号传导途径中的分子机制研究打下基础。
1 材料与方法
试验于 2015—2016年在中国农业科学院果树研究所完成。
1.1 供试菌株及培养条件
苹果炭疽叶枯病菌(G. cingulata)强致病力菌株W16,由笔者课题组2014年分离自辽宁省绥中市一个病害重发生果园[5],W16和由其衍生的转化子采用PDA 培养基进行培养。农杆菌(Agrobacterium tumefacien)菌株 LBA4404(由云南农业大学何月秋教授惠赠)采用LB培养基进行培养,用于ATMT。大肠杆菌(Escherichia coli)菌株TG1采用LB培养基进行培养,用作质粒的宿主。为检测GcAP1β的表达模式,RNA提取样品分别取自菌丝(PDA培养3 d的菌丝体)、分生孢子(无菌蒸馏水刮洗 PDA平板10 d的培养物,2层擦镜纸过滤除去菌丝后,离心收集分生孢子)、芽管(用毛笔将106个/mL分生孢子悬浮液涂在叶片正面,28℃条件下保湿培养6 h后,用灭菌刀片轻刮叶子叶面,收集萌发的分生孢子)、附着胞(方法同芽管,保湿培养10 h,收集叶片表面形成的附着胞)和叶片接种48 h(方法同芽管,保湿培养 48 h,将整个叶片液氮速冻后研磨)。为检测CgPG1、CgPG2、pnl-1、pnl-2、pelA和pelB在GcAP1β突变体中的表达量,RNA提取样品分别取自PDA培养6 d的菌丝体。
1.2 核酸操作

表 1 PCR引物序列Table 1 The primers used in this study
1.3 突变体A7346 T-DNA右翼序列分析
以质粒pCamhybgfp1(GenBank accession number:KX223837)为模板,用引物H-F1和H-F2进行PCR扩增,产物纯化后作为探针(P1)进行A7346 T-DNA插入拷贝数的Southern blot分析。A7346 T-DNA右翼序列采用hiTAIL-PCR方法[26]进行PCR扩增,产物连结到pUCm-T载体(生工生物),由北京三博远志生物技术有限责任公司测序。获得的 T-DNA右翼序列参考围小从壳菌菌株23基因组(http://genome.jgi.doe. gov/Gloci1/Gloci1.home.html),进行本地blast分析。目的GcAP1复合体β亚基基因(GcAP1β)利用比较真菌基因组平台[27]进一步进行分析,并提取基因结构及其上下游序列和蛋白序列信息。
1.4 GcAP1复合体β亚基基因的敲除与恢复
以野生型菌株W16基因组为模板,用引物S2Dr1和S3S1扩增GcAP1β基因上游序列,SalⅠ/SpeⅠ酶切后,连结到SalⅠ/SpeⅠ酶切的pCambiaMX9(GenBank accession number:KX755248),生成质粒pCamS1。再以野生型菌株W16基因组为模板,用引物S2G2和S2DF2扩增GcAP1β基因下游序列,EcoRⅠ/SalⅠ酶切后,连结到 EcoRⅠ/SalⅠ酶切的 pCambiaMX9(GenBank accession number:KX755248),生成质粒pCamS2。约2.0 kb的潮霉素抗性基因(hph)序列从质粒pTFCM[28](由华中农业大学姜道宏教授惠赠)SalⅠ酶切得到后,连结到SalⅠ酶切的pCamS2,形成GcAP1β基因敲除载体 pCamS2KN1(图 1)。pCamS2KN1被电转到农杆菌 LBA4404中后,利用ATMT方法[29]将GcAP1β从W16基因组中敲除。筛选潮霉素B(生工生物,终浓度100 μg·mL-1)抗性转化子,对假定的GcAP1β基因突变体用引物HB1和S2R1进行特定位点的PCR扩增分析(图1),并以探针P2(以野生型菌株W16基因组为模板,由引物P-f和P-r进行PCR扩增及纯化获得)进行Southern blot鉴定。以 W16基因组为模板,用引物 HB1和 HB2扩增GcAP1β及其假定的启动子序列,BamHⅠ/SalⅠ酶切后,连结到载体 pGapneoR12(GenBank accession number:KY363244),生成的GcAP1β基因恢复载体pCamGcAP1β(形成GcAP1β-gfp融合表达结构)被电转到农杆菌LBA4404中,利用ATMT[29]将GcAP1β-gfp融合基因转移到Δgcap1β突变体基因组中,筛选G418(生工生物,终浓度500 μg·mL-1)抗性转化子,对假定的GcAP1β基因恢复菌株用引物S2F和S2R进行PCR扩增分析和GFP信号检测(DM5000B型荧光显微镜,德国Leica)进行确定。

图 1 GcAP1β的克隆和鉴定Fig. 1 The cloning and identification of GcAP1β
1.5 表型分析
真菌的生长速度和产孢能力测验采用WU等[30]的方法进行。挑取气生菌丝接种于PDA平板上(直径9 cm),培养6 d后,测量菌落直径;平板继续培养至10 d,用10 mL无菌水刮洗平板,用擦镜纸滤去菌丝后,采用血球计数板进行测定,计算每毫升孢子悬浮液中的产孢量。分生孢子的萌发、附着胞的形成能力测验采用XU等[31]的方法。将无菌的疏水载玻片至于水琼脂(8%)平板上,将10 μL分生孢子悬浮液(104个/mL)滴于载玻片,28℃黑暗条件下,保湿培养至8 h,观测生孢子萌发情况,保湿培养至20 h,观测附着胞形成情况。每个重复观测100个分生孢子。计算公式:孢子萌发率(%)=萌发的孢子数/检测的孢子总数×100;附着胞形成率(%)=形成附着胞的萌发孢子数/检测的萌发孢子总数×100。
叶片致病性测定:摘取成熟度一致的金冠(感病品种)叶片,无菌水冲洗后,用2 μL分生孢子悬浮液(1×105个/mL)滴在叶片正面,每个叶片接种6个悬滴,接种后,置于塑料盒中冷凝的10%水琼脂上。28℃条件下,黑暗培养10 h后,转入光照14 h,以此循环。每天观察发病情况。苹果致病性测定:苹果果实被75%酒精消毒后,用牙签刺约3 mm深的伤口,2 μL分生孢子悬浮液(1×105个/mL)接种于伤口处,28℃条件下,接种10 d后观测果实发病情况。野生型菌株W16作为阳性对照。每个处理重复3次,用DPS软件进行差异显著性分析。
优质的思想政治教育是培养高素质人才的保障,加强思想政治教育的研究,探讨思想政治教育的有效途径,才能使思想政治教育取得好的效果,才能使大学生思想政治素质得到提高,进而使大学生符合社会发展的要求。经过社会、教育者和大学生的共同努力,相信思想政治教育会朝着更好的方向发展,大学生的思想政治素质会得到更大地提高。
1.6 生物信息学分析
应用ClustalX对GcAP1β基因序列及其cDNA序列进行比对,以确定GcAP1β基因内含子的有无。应用 InterProScan[32-33]对 GcAP1β进行保守结构域(Conserved Domain)分析。应用 SignalP 4.1[34]对 GcAP1β的N端进行信号肽分析。应用ProtComp 9.0(http://www.softberry.com)对GcAP1β进行亚细胞定位分析。应用TMHMM Server v. 2.0(http://www.cbs. dtu.dk/services/TMHMM/)对GcAP1β进行跨膜螺旋结构分析。
2 结果
2.1 GcAP1β的克隆与分析
Southern blot分析结果表明,T-DNA插入突变体A7346经过酶切的泳道只杂交出一个条带,推测其表型是由T-DNA以单拷贝插入至基因组所致(图2)。运用hiTail-PCR技术克隆得到的T-DNA右翼序列与围小从壳菌菌株23基因组进行比对,发现T-DNA插入位点位于一个注释但功能尚未解析的基因(e_gw1.11.622.1)的第一个外显子内。InterProScan分析显示该基因蛋白序列含有典型的衔接蛋白AP1复合体 β亚基结构域(IPR026739)。因此,将这一基因命名为 GcAP1β(GcAP1复合体 β亚基基因)。GcAP1β基因全长2 321 bp,含有3个内含子,编码720个氨基酸。SignalP预测该基因蛋白序列无信号肽,表明其可能不是分泌蛋白。

图 2 T-DNA插入突变体A7346 Southern杂交分析Fig. 2 Southern hybridization analysis of T-DNA insertional mutant A7346
2.2 Δgcap1β突变体和GcAP1β基因恢复菌株的分子鉴定
特定位点的PCR检测结果(图3-A)显示,Δgcap1β突变体Δgcap1β-1、Δgcap1β-2和Δgcap1β-3基因组中的GcAP1β均被敲除。Southern blot分析结果显示,Δgcap1β-1、Δgcap1β-2和Δgcap1β-3均杂交出一条带,而野生型菌株 W16杂交出与敲除突变体长度不同的一条带(图3-B),表明外源的hph在Δgcap1β突变体基因组中为单位点、单拷贝的插入,也证实GcAP1β被hph替换。RT-PCR检测结果(图3-C)表明,GcAP1β被导入到突变体Δgcap1β-1基因组中,且能在恢复菌株Δgcap1β-GcAP1β中表达。
2.3 GcAP1β缺失对菌丝生长的影响
在PDA平板上,与野生型菌株W16相比,Δgcap1β突变体Δgcap1β-1、Δgcap1β-2和Δgcap1β-3菌落成褶皱状,菌丝生长速度明显减慢(图4、表2),但分生孢子产量、分生孢子萌发率、附着胞形成率则无显著差异(表2)。
2.4 GcAP1β的缺失对致病力的影响
致病测验结果显示,Δgcap1β在苹果叶片上引起极小的点状斑,与A7346极为相似,然而同条件下,W16引起明显的坏死斑(图5-A);Δgcap1β在苹果果实上引起较小的凹陷斑,而W16引起的病斑明显比Δgcap1β大的多(图5-B)。此外,GcAP1β基因恢复菌株Δgcap1β-GcAP1β完全修复了因GcAP1β基因缺失造成的表型缺陷(图5、表2)。这些结果表明GcAP1β的缺失导致苹果炭疽叶枯病菌菌丝生长减慢和致病力降低。表 2 苹果炭疽叶枯病菌野生型菌株及其衍生的转化子表型分析

图 3 Δgcap1β突变体和GcAP1β基因恢复菌株的分子鉴定Fig. 3 Molecular confirmation of the Δgcap1β mutants and the GcAP1β complementation strain Δgcap1β-GcAP1β

图4 苹果炭疽叶枯病菌野生型菌株及其衍生的转化子菌落形态Fig. 4 Colony morphology of G. cingulata strain W16 and its derived transformants

图5 苹果炭疽叶枯病菌野生型菌株及其衍生的转化子致病力测定Fig. 5 Pathogenicity assay of G. cingulata strain W16 and its derived transformants
Table 2 Phenotypic analysis of G. cingulata strain W16 and its derived transformants

**表示在P=0.01水平与野生型的差异显著** represented statistically significant difference compared with W16, P=0.01
2.5 GcAP1β亚细胞定位
TMHMM Server v. 2.0分析结果表明,GcAP1β不具有跨膜螺旋结构;ProtComp 9.0分析结果表明,GcAP1β没有明确的定位信号。为了确定该蛋白的亚细胞定位,构建了GcAP1β-gfp融合表达的GcAP1β恢复菌株 Δgcap1β-GcAP1β。荧光显微镜下检测结果表明,融合蛋白 GcAP1β-GFP分布于细胞质中,与T-DNA插入突变体A7346中GFP蛋白(gfp的表达由GAPDH启动子控制)分布特点相似(图6)。

图6 融合蛋白GcAP1β-GFP分布于细胞质中Fig. 6 The fused protein GcAP1β-GFP is distributed to cytoplasm
2.6 GcAP1β在苹果炭疽叶枯病菌发育各阶段表达情况qRT-PCR检测结果表明,GcAP1β在苹果炭疽叶枯病菌各个发育阶段均有表达。相对于菌丝阶段(PDA培养3 d时)的表达量,GcAP1β在分生孢子、芽管和附着胞阶段表达量均有不同程度的降低,而在侵染后(叶片接种 48 h)表达量却明显提高(图7)。

图 7 GcAP1β在各发育阶段的表达情况Fig. 7 The expression of GcAP1β at different development stages
2.7 GcAP1β的缺失对CgPG1、CgPG2、pnl-1、pnl-2、pelA和pelB表达量的影响
由于Δgcap1β突变体导致苹果炭疽叶枯病菌致病力的降低(图7),笔者假设Δgcap1β突变体中果胶酶相关基因的转录受阻。因此,利用qRT-PCR技术检测了CgPG1、CgPG2、pnl-1、pnl-2、pelA和pelB在 Δgcap1β突变体中的表达情况。相比于野生型菌株W16,在 Δgcap1β突变体中,CgPG1表达量降低至20.3%,CgPG2表达量降低至16.5%,pnl-1表达量降低至8.2%,pnl-2表达量降低至14.4%,pelA表达量降低至4.4%,pelB表达量降低了至0.8%(图8)。这些结果显示GcAP1β正调控基因CgPG1、CgPG2、pnl-1、pnl-2、pelA和pelB的表达。
3 讨论
真菌菌丝的生长与囊泡(vesicles)的营养运输密切相关[35]。本研究结果表明,GcAP1复合体β亚基的缺失导致苹果炭疽叶枯病菌菌丝生长缓慢;qRT-PCR检测结果表明,GcAP1β在菌丝生长阶段表达量相对较高(图 7)。值得注意的是,在哺乳动物中,衔接蛋白 AP-1复合体负责运输蛋白至网格蛋白包被的囊泡(clathrin-coated vesicles)中[18,36]。因此,笔者推测真菌菌丝的生长与 GcAP1复合体负责运输蛋白至囊泡的功能密切相关。胶胞炭疽菌侵入寄主后,先保持活体营养型生长,然后转为死体营养型生长[30]。苹果炭疽叶枯病菌发病速度特别快,从侵染叶片到发病落叶仅需3—4 d[37]。本研究中,苹果炭疽叶枯病菌叶片接种48 h时已出现病斑。qRT-PCR检测结果显示,GcAP1复合体β亚基基因在苹果炭疽叶枯病菌各个发育阶段均有表达,但是以侵染后(叶片接种48 h时)表达量相对最高(图7)。这些结果说明,GcAP1复合体β亚基基因在苹果炭疽叶枯病菌死体营养型生长阶段中发挥着重要作用。
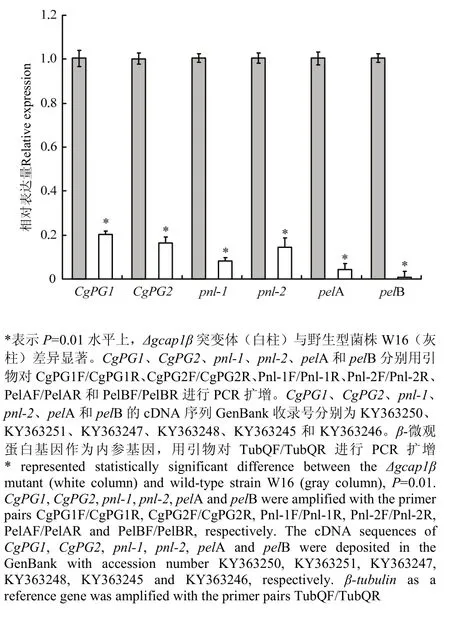
图 8 Δgcap1β突变体CgPG1、CgPG2、pnl-1、pnl-2、pelA和pelB的表达量分析Fig. 8 The expression analyses of CgPG2, pnl-1, pnl-2, pelA and pelB in the Δgcap1β mutant
在植物病原真菌中,由附着胞调节的侵入寄主方式在其致病过程起到重要作用。在炭疽菌中,丝裂原活化蛋白激酶(Mitogen-activated protein kinase,MAPK)级联信号途径调控其分生孢子的萌发、附着胞的形成[9]。在酿酒酵母(Saccharomyces cerevisiae)中,衔接蛋白Ste50与G蛋白有关的Cdc42-Ste20激酶复合体互作后,激活MAPKKK基因Ste11的转录,进而激活 MAPK级联信号传递链[38]。在胶胞炭疽菌中,MAPK途径基因中的CgMEK1或Cgl-SLT2的缺失,导致萌发的分生孢子不能形成附着胞。本研究中,GcAP1复合体β亚基基因的缺失对分生孢子的萌发、附着孢的形成无影响(表2)。这一结果暗示,GcAP1复合体可能对基因CgMEK1和Cgl-SLT2的转录无直接或间接的影响。
在植物病原菌和寄主相互作用中,病原菌分泌果胶酶降解寄主细胞壁的果胶聚合体,促进病原菌的侵入和定殖[10-14]。此外,病原菌的果胶酶分泌量不仅决定其致病力的强弱,还决定其致害的症状[39]。因为Δgcap1β突变体仅在苹果叶片上引起极小的点状斑以及在苹果果实(有伤接种)上引起较小的凹陷斑(图5),笔者假设Δgcap1β突变体果胶酶有关基因(CgPG1、CgPG2、pnl-1、pnl-2、pelA和 pelB)转录可能受阻。qRT-PCR检测结果表明,Δgcap1β突变体果胶酶有关基因的表达量与野性型菌株的相比明显降低(图8)。这些结果表明,Δgcap1β突变体致病力下降的一个重要原因是,GcAP1蛋白β亚基基因的缺失阻遏果胶酶有关基因的表达。但是,GcAP1蛋白是否直接或间接调控果胶酶有关基因的转录,有待进一步研究。
4 结论
衔接蛋白 GcAP1复合体分布于细胞质中。GcAP1β在分生孢子、芽管和附着胞阶段均表达,但表达量明显低于菌丝生长阶段,是苹果炭疽叶枯病菌生长发育所需要的。GcAP1调控 CgPG1、CgPG2、pnl-1、pnl-2、pelA和pelB的表达,是苹果炭疽叶枯病菌一个重要的毒力因子。
[1] JACKSON L P, KELLY B T, MCCOY A J, GAFFRY T, JAMES L C, COLLINS B M, HONING S, EVANS P R, OWEN D J. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell, 2010, 141(7):1220-1229.
[2] 李保华, 王彩霞, 董向丽. 我国苹果主要病害研究进展与病害防治中的问题. 植物保护, 2013, 39(5): 46-54.
LI B H, WANG C X, DONG X L. Research progress in apple diseases and problems in the disease management in China. Plant Protection, 2013, 39(5): 46-54. (in Chinese)
[3] WANG C X, ZHANG Z F, LI B H, WANG H Y, DONG X L. First report of Glomerella leaf spot of apple caused by Glomerella cingulata in China. Plant Disease, 2012, 96(6): 912.
[4] 任斌, 高小宁, 韩青梅, 黄丽丽. 苹果炭疽叶枯病病原 Glomerella cingulata及其侵染过程. 植物保护学报, 2014, 41(5): 608-614.
REN B, GAO X N, HAN Q M, HUANG L L. Etiology and infection process of Glomerella cingulata causing Glomerella leaf spot of apple. Acta Phytophylacica Sinica, 2014, 41(5): 608-614. (in Chinese)
[5] 张俊祥, 吴建圆, 冀志蕊, 迟福梅, 蒋晓玲, 董庆龙, 周宗山. 农杆菌介导的苹果炭疽叶枯病菌遗传转化及插入突变体的筛选. 基因组学与应用生物学, 2014, 33(6): 1261-1267.
ZHANG J X, WU J Y, JI Z R, CHI F M, JIANG X L, DONG Q L, ZHOU Z S. Agrobacterium tumefaciens-mediated transformation of Glomerella cingulata and screening pathogenicity-deficient mutants. Genomics and Applied Biology, 2014, 33(6): 1261-1267. (in Chinese)
[6] GONZÁLEZ E, SUTTON T B. Population diversity within isolates of Colletotrichum spp. causing Glomerella leaf spot and bitter rot of apples in three orchards in north Carolina. Plant Disease, 2004, 88(12): 1335-1340.
[7] VELHO A C, STADNIK M J, CASANOVA L, MONDINO P, ALANIZ S. First report of Colletotrichum karstii causing Glomerella leaf spot on apple in Santa Catarina State, Brazil. Plant Disease, 2014, 98(1): 157.
[8] 王薇, 符丹丹, 张荣, 孙广宇. 苹果炭疽叶枯病病原学研究. 菌物学报, 2015, 34(1): 13-25.
WANG W, FU D D, ZHANG R, SUN G Y. Etiology of apple leaf spot caused by Colletotrichum spp. Mycosystema, 2015, 34(1): 13-25. (in Chinese)
[9] KUBO Y, TAKANO Y. Dynamics of infection-related morphogenesis and pathogenesis in Colletotrichum orbiculare. Journal of General Plant Pathology, 2013, 79(4): 233-242.
[10] CENTIS S, DUMAS B, FOURNIER J, MAROLDA M, ESQUERRE-TUGAYE M T. Isolation and sequence analysis of Clpg1, a gene coding for an endopolygalacturonase of the phytopathogenic fungus Colletotrichum lindemuthianum. Gene, 1996, 170(1): 125-129.
[11] LI J, GOODWIN P H. Expression of cgmpg2, an endopolygalacturonase gene of Colletotrichum gloeosporioides f. sp. malvae, in culture and during infection of Malva pusilla. Journal of Phytopathology, 2002, 150(4/5): 213-219.
[12] SHIH J, WEI Y, GOODWIN P H. A comparison of the pectate lyase genes, pel-1 and pel-2, of Colletotrichum gloeosporioides f. sp. malvae and the relationship between their expression in culture and during necrotrophic infection. Gene, 2000, 243(1/2): 139-150.
[13] WEI Y D, SHIH J, LI J R, GOODWIN P H. Two pectin lyase genes, pnl-1 and pnl-2, from Colletotrichum gloeosporioides f. sp. malvae differ in a cellulose-binding domain and in their expression during infection of Malva pusilla. Microbiology, 2002, 148: 2149-2157.
[14] YAKOBY N, BENO-MOUALEM D, KEEN N T, DINOOR A, PINES O, PRUSKY D. Colletotrichum gloeosporioides pelB is an important virulence factor in avocado fruit-fungus interaction. Molecular Plant-Microbe Interactions, 2001, 14(8): 988-995.
[15] ALKAN N, MENG X, FRIEDLANDER G, REUVENI E, SUKNO S, SHERMAN A, THON M, FLUHR R, PRUSKY D. Global aspects of pacC regulation of pathogenicity genes in Colletotrichum gloeosporioides as revealed by transcriptome analysis. Molecular Plant-Microbe Interactions, 2013, 26(11): 1345-1358.
[16] OHNO H. Clathrin-associated adaptor protein complexes. Journal of Cell Science, 2006, 119(18): 3719-3721.
[17] OWEN D J, COLLINS B M, EVANS P R. Adaptors for clathrin coats: structure and function. Annual Review of Cell and Developmental Biology, 2004, 20: 153-191.
[18] BOEHM M, BONIFACINO J S. Adaptins - The final recount. Molecular Biology of the Cell, 2001, 12(10): 2907-2920.
[19] HIRST J, BARLOW L D, FRANCISCO G C, SAHLENDER D A, SEAMAN M N J, DACKS J B, ROBINSON M S. The fifth adaptor protein complex. PLoS Biology, 2011, 9(10): e1001170.
[20] ROBINSON M S, BONIFACINO J S. Adaptor-related proteins. Current Opinion in Cell Biology, 2001, 13(4): 444-453.
[21] ROBINSON M S, SAHLENDER D A, FOSTER S D. Rapid inactivation of proteins by rapamycin-induced rerouting to mitochondria. Developmental Cell, 2010, 18(2): 324-331.
[22] HE Y Q. An improved protocol for fungal DNA preparation. Mycosystema, 2000, 19(3): 434.
[23] ZHANG J X, WU X X, BI Y Q, WU Y X, LIN G H, HE Y Q, MAO Z C. First report of Fusarium proliferatum infecting carnation (Dianthus caryophyllus L.) in China. Journal of Phytopathology, 2013, 161(11/12): 850-854.
[24] ZHANG J X, WU Y X, HO H H, ZHANG H, HE P F, HE Y Q. BZcon1, a SANT/Myb-type gene involved in the conidiation of Cochliobolus carbonum. G3-Genes Genomes Genetics 2014, 4(8):1445-1453.
[25] LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCtmethod. Methods, 2001, 25(4): 402-408.
[26] LIU Y G, CHEN Y. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques, 2007, 43(5): 649-656.
[27] CHOI J, CHEONG K, JUNG K, JEON J, LEE G W, KANG S, KIM S, LEE Y W, LEE Y H. CFGP 2.0: a versatile web-based platform for supporting comparative and evolutionary genomics of fungi and Oomycetes. Nucleic Acids Research, 2013, 41: D714-D719.
[28] LI M X, GONG X Y, ZHENG J, JIANG D H, FU Y P, HOU M S. Transformation of Coniothyrium minitans, a parasite of Sclerotinia sclerotiorum, with Agrobacterium tumefaciens. FEMS Microbiology Letters, 2005, 243(2): 323-329.
[29] LEE M H, BOSTOCK R M. Agrobacterium T-DNA-mediated integration and gene replacement in the brown rot pathogen Monilinia fructicola. Current Genetics, 2006, 49(5): 309-322.
[30] WU J, JI Z, WANG N, CHI F, XU C, ZHOU Z, ZHANG J. Identification of conidiogenesis-associated genes in Colletotrichum gloeosporioides by Agrobacterium tumefaciens-mediated transformation. Current Microbiology, 2016, 73(6): 802-810.
[31] XU J R, URBAN M, SWEIGARD J A, HAMER J E. The CPKA gene of Magnaporthe grisea is essential for appressorial penetration. Molecular Plant-Microbe Interactions, 1997, 10(2): 187-194.
[32] HUNTER S, JONES P, MITCHELL A, APWEILER R, ATTWOOD T K, BATEMAN A, BERNARD T, BINNS D, BORK P, BURGE S, DE CASTRO E, COGGILL P, CORBETT M, DAS U, DAUGHERTY L, DUQUENNE L, FINN RD, FRASER M, GOUGH J, HAFT D, HULO N, KAHN D, KELLY E, LETUNIC I, LONSDALE D, LOPEZ R, MADERA M, MASLEN J, MCANULLA C, MCDOWALL J, MCMENAMIN C, MI H Y, MUTOWO-MUELLENET P, MULDER N, NATALE D, ORENGO C, PESSEAT S, PUNTA M, QUINN AF, RIVOIRE C, SANGRADOR-VEGAS A, SELENGUT JD, SIGRIST C J A, SCHEREMETJEW M, TATE J, THIMMAJANARTHANAN M, THOMAS P D, WU C H, YEATS C, YONG S Y. InterPro in 2011: new developments in the family and domain prediction database. Nucleic Acids Research, 2012, 40: D306-D312.
[33] MITCHELL A, CHANG H Y, DAUGHERTY L, FRASER M, HUNTER S, LOPEZ R, MCANULLA C, MCMENAMIN C, NUKA G, PESSEAT S, SANGRADOR-VEGAS A, SCHEREMETJEW M, RATO C, YONG SY, BATEMAN A, PUNTA M, ATTWOOD TK, SIGRIST C J A, REDASCHI N, RIVOIRE C, XENARIOS I, KAHN D, GUYOT D, BORK P, LETUNIC I, GOUGH J, OATES M, HAFT D, HUANG H Z, NATALE DA, WU C H, ORENGO C, SILLITOE I, MI HY, THOMAS P D, FINN R D. The InterPro protein families database: the classification resource after 15 years. Nucleic Acids Research, 2015, 43: D213-D221.
[34] PETERSEN T N, BRUNAK S, VON HEIJNE G, NIELSEN H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods, 2011, 8(10): 785-786.
[35] STEINBERG G. Hyphal growth: a tale of motors, lipids, and the Spitzenkorper. Eeukaryotic Cell, 2007, 6(3): 351-360.
[36] ROBINSON M S, SAHLENDER D A, FOSTER S D. Rapid inactivation of proteins by rapamycin-induced rerouting to mitochondria. Developmental Cell, 2010, 18(2): 324-331.
[37] 吴建圆, 冀志蕊, 李壮, 程存刚, 周宗山, 张俊祥. nptⅡ基因真菌表达载体的构建及在苹果炭疽叶枯病菌遗传转化中的应用. 基因组学与应用生物学, 2015, 34(10): 2156-2160.
WU J Y, JI Z R, LI Z, CHENG C G, ZHOU Z S, ZHANG J X. Construction of the fungus expression vector of nptⅡ gene and applying to the genetic transformation in Glomerella cingulata. Genomics and Applied Biology, 2015, 34(10): 2156-2160. (in Chinese)
[38] RAMEZANI-RAD M. The role of adaptor protein Ste50-dependent regulation of the MAPKKK Ste11 in multiple signalling pathways of yeast. Current Genetics, 2003, 43(3): 161-170.
[39] REIGNAULT P H, KUNZ C, DELAGE N, MOREAU E, VEDEL R, HAMADA W, BOMPEIX G, BOCCARA M. Host- and symptomspecific pectinase isozymes produced by Botrytis cinerea. Mycological Research, 2000, 104(4): 421-428.
(责任编辑 岳梅)
Gene Cloning and Functional Analysis of GcAP1 Complex Beta Subunit in Glomerella cingulata
ZHANG JunXiang, JI ZhiRui, WANG Na, XU ChengNan, CHI FuMei, ZHOU ZongShan
(Research Institute of Pomology, Chinese Academy of Agricultural Sciences, Xingcheng 125100, Liaoning)
【Objective】 The objectives of this study are to determine the function of β subunit of adaptor protein GcAP1 complex in growth and pathogenicity of Glomerella leaf spot of apple pathogen Glomerella cingulata, investigate expression patterns of the GcAP1β in the fungal growth and pathogenicity, decipher whether or not GcAP1β regulate the expression of endopolygalacturonase genes CgPG1 and CgPG2, pectin lyase genes pnl-1 and pnl-2, pectate lyase genes pelA and pelB, and to lay a foundation for further studies of adaptor protein in pathogenic signal transduction pathways of G. cingulata.【Method】 Based on theGcAP1β deletion vector and GcAP1β-gfp fused expression vector, the Δgcap1β mutant and the GcAP1β complementation strain Δgcap1β-GcAP1β were structured using ATMT, respectively, verified by RT-PCR and Southern blot analysis. Colony growth rate, sporulation, germination rate, appressorial formation rate and pathogenicity of the Δgcap1β mutant and the GcAP1β complementation strain Δgcap1β-GcAP1β were assayed, compared with the wild-type strain W16. GcAP1β subcellular localization was carried out with the bioinformatics softwares ProtComp 9.0 and TMHMM, along with signal observation of GcAP1β-GFP. The GcAP1β expression levels in hyphae, conidia, appressoria and pathogenicity stage were identified by qRT-PCR. Moreover, the expression levels of CgPG1, CgPG2, pnl-1, pnl-2, pelA and pelB in the wild-type strain W16 and the Δgcap1β mutant were detected, respectively. 【Result】 GcAP1β is 2 321 bp in length, including 3 introns, which encodes a 720 amino acids. Compared with the wild-type strain W16, the Δgcap1β mutant showed a rill-like fold colony and decreased growth, while sporulation, germination rate and appressorial formation rate were unaffected. Virulence of the Δgcap1β mutant reduced significantly, which induced tiny spots on the leaves. Moreover, the GcAP1β complementation strain Δgcap1β-GcAP1β fully restored the phenotype flaws by reintroducing GcAP1β to the Δgcap1β mutant. Fluorescent signal showed that the fused protein GcAP1β-GFP was distributed to the cytoplasm. qRT-PCR analysis showed that GcAP1β expresses through the lifecycle of G. cingulata, and the highest expression level of GcAP1β occurred at the post-invasion to leaves. Compared with WT, the Δcgap1β mutant showed a drastic reduction of CgPG1 transcripts (20.3%), CgPG2 transcripts (16.5%), pnl-1 transcripts (8.2%), pnl-2 transcripts (14.4%), pelA transcripts (4.4%) and pelB transcripts (0.8%). 【Conclusion】 The adaptor protein GcAP1 complex is distributed to the cytoplasm and is necessary for growth and development of G. cingulata; GcAP1 regulates the expression of CgPG1, CgPG2, pnl-1, pnl-2, pelA and pelB and is a vital virulence factor of G. cingulata.
adaptor protein; pectinase; apple; Colletotrichum; virulence
2016-12-26;接受日期:2017-02-06
国家自然科学基金青年科学基金(31501596)、中央级公益性科研院所基本科研业务费专项(1610182016002)、中国农业科学院科技创新工程
联系方式:第一作者、共同通信作者张俊祥,E-mail:zhangjunxiang@caas.cn。通信作者周宗山,E-mail:zszhouqrj@163.com

