Patterns offorest composition and their long term environmentaldrivers in the tropicaldry forest transition zone of southern Africa
Vera De Cauwer,Coert J.Geldenhuys,Raf Aerts,Miya Kabajaniand Bart Muys
Patterns offorest composition and their long term environmentaldrivers in the tropicaldry forest transition zone of southern Africa
Vera De Cauwer1,2*,Coert J.Geldenhuys3,Raf Aerts2,4,Miya Kabajani1and Bart Muys2
Background:Tropicaldry forests cover less than 13%of the world’s tropicalforests and their area and biodiversity are declining.In southern Africa,the major threat is increasing population pressure,while drought caused by climate change is a potentialthreat in the drier transition zones to shrub land.Monitoring climate change impacts in these transition zones is difficult as there is inadequate information on forest composition to allow disentanglement from other environmentaldrivers.
Baikiaea woodland,Tree community,Namibia,boosted regression trees,Pterocarpus angolensis, Disturbance,Miombo Ecoregion,Climate change
Background
Tropicaldry forests representabout 8 to 13%ofthe world’s tropical forests(Hansen et al.2013).They provide important ecosystem services such as climate regulation and carbon storage,and support millions of poor people through timber and non-timber forest products(Snyder et al.2004; Shackleton et al.2007;Blackie et al.2014).They are an important safety net for human and animal populations by providing food during dry years(Chidumayo and Gumbo 2010).Virtually allremaining tropicaldry forests are threatened by deforestation and degradation and should be given high conservation priority(Miles et al.2006;Hansen et al. 2013).However,they are among the least studied forest ecosystems hampering sustainable forest management and adequate forest policy(Blackie etal.2014).
Increasing human populations are the main threat to tropical dry forests in Africa,inducing changes in land use practices,land cover and fire regimes(Miles et al. 2006;Leadley 2010;Cabral et al.2011;Sloan and Sayer 2015).The impact of global change on Africa’s dry forests is unclear as predicted climate changes vary at a regional scale while increasing CO2and drought can have opposite effects on tree cover(Hély et al.2006; Lucht et al.2006;Thuiller et al.2006;Leadley 2010;Liu et al.2013).Transition zones from open dry forest to shrub and grassland can expect the largest impact,particularly where mean annual rainfall has a pronounced effect on woody cover(Sankaran et al.2005,2008) (Fig.1).More drought combined with increasing fire frequency(Pricope and Binford 2012)and population pressure,may induce changes towards other vegetation types and thereby affect human communities depending on forest resources.
One of the moderate climate change scenarios,the B1 emission scenario which can best be compared by the Representative Concentration Pathway 4.5(IPCC 2007; Rogelj et al.2012;IPCC 2014),predicts the biggest decrease in rainfall for the African dry forest transition zones in northern Namibia,southern Angola,southeastern Botswana,and the north-west of South Africa (Fig.1).This would reduce the range of deciduous broad-leaved trees(Thuiller et al.2006).Monitoring forest composition in transition zones can provide an early warning system for climate change impacts.However, there is inadequate information on the current state of those forest ecosystems to disentangle climate change from other environmentaleffects.The lack of information hampers the development of climate change adaptation strategies,informed forest management and land use planning decisions.The use of passive remote sensing offers only limited options because the spectral signal from the open forests is influenced by fire scars,shrub and grass cover,which all have a high intra-and inter-annual variability(FAO 2001;Ganzin et al.2005;De Cauwer 2015).
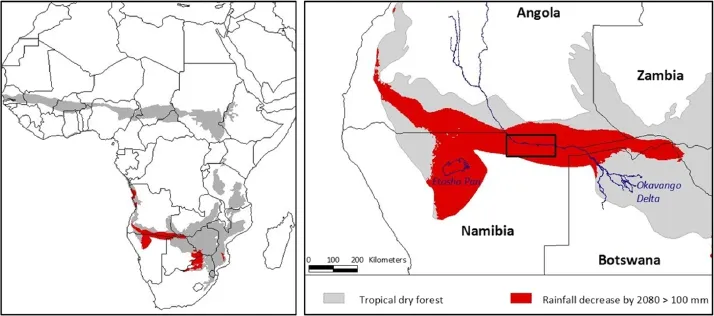
Fig.1 Left:Tropicaldry forest ecozone in Africa(FAO 2000)with areas susceptible to drought.Susceptible areas are situated in the transition zone to shrub land where woody vegetation cover is mainly influenced by mean annualprecipitation(MAP < 650 mm)(Sankaran et al.2005) and where the largest decreases(>15%)in MAP are predicted.Right:Study area in the dry tropicalforest transition zone.Future MAP is based on the B1 SRES emission scenario for 2080(HADCM3 model,WorldClim)
This study aims to provide more insight in the forest composition of a tropical dry forest transition zone in southern Africa where a large reduction in rainfall is predicted,i.e.the open Baikiaea forests at the southwestern edge of the Miombo ecoregion(Fig.1).Forest composition here is different from the wetter Miombo woodlands more northwards,and has a lower human population density.The woodlands contribute directly tonationaleconomies through ecotourism focused on large mammals,and the provision of fuel,construction wood and fodder(Barnes et al.2010).Some detailed studies on forest composition in this area focused on smaller study areas(Childes and Walker 1987;Strohbach and Petersen 2007;Aarrestad et al.2011;Strohbach 2013;Revermann and Finckh 2013).Few studies have examined the longterm changes in the composition of late successionalforest(e.g.Mosugelo et al.2002).Detecting forest patterns in the study area is complicated by 1)a lack of obvious environmental gradients with exception of a few rivers and dunes and 2)the difficulty to distinguish tree communities with small datasets(<200 plots)(Childes and Walker 1987;Strohbach 2013;De Cauwer 2013).Regional studies indicate that forest dynamics are mainly driven by fire,drought and browsing(Burke 2006;Archibald et al. 2009;Fanshawe 2010).The long-term effect of fire on forest composition remains however controversial,especially because it is influenced by other variables,such as soil, browsing and rainfall(Bond et al.2003;Sankaran et al. 2008).The impact of elephant populations on woody vegetation is well studied in the eastern Baikiaea woodlands(Makhabu et al.2006;Holdo 2007;Loarie et al. 2009;Kalwij et al.2010;Aarrestad et al.2011),however, elephants are less common in the western parts and cattle are the most important large herbivores.
This study combines data of different forest inventories collected over four decades to identify tree communities over a large area and study their long-term interactions with site variables,including disturbances.Larger trees formed the basis of the study as their abundance accumulates the effect of past climate variation(Thijs et al.2014; Zhu et al.2014).The study aims at 1)distinguishing late succession tree communities in the western Baikiaea woodlands and describing their composition;2)determining which environmental variables including disturbances have shaped the communities and their species composition;and 3)detecting trends in the composition of the tree communities over the last40 years.
Methods
Study area
The study area in the Baikiaea woodlands of Namibia and Angola covers 21,000 km2(Fig.1).Most of the area is communal land with some parts managed as community forests or state forest.The study area is situated within the Kavango Zambezi Trans-frontier Conservation Area(KAZA TFCA),the largest trans-frontier conservation area in the world(WWF 2012).The generally flat landscape has very gradual slopes.The main topographic feature is the Okavango river valley and its tributaries of dry,fossil rivers.The south-western part of the study area has permanent parallel dunes,only a few meters higher than the surrounding areas(Graz 1999).
The soil consists of aeolian sediments forming Arenosols,with exception of the floodplains and fossil rivers where clay and silt contents are higher.The sandy soils have low levels of nutrients,particularly carbon and nitrogen(Gröngröft et al.2013).Mean annual rainfall is about 560 mm,with increasing rainfall towards the north.Seasonal rainfall variability is high with most rain in the wettest months of the rainy season,between November and April.More details on the physical environment of the study area are given in Table 1.
The vegetation outside the Okavango river valley is open forest according to FAO definitions,with an average canopy cover of25%and canopy heights of 10 to 15 m.It is characterised by a low growing stock(Table 1)and tree species Pterocarpus angolensis,Baikiaea plurijuga,Burkea africana and Schinziophyton rautanenii.In Namibia,the vegetation is referred to as Northern Kalahari dry forests and woodlands(Giess 1998)and in Angola as B.plurijuga woodland savannah with stands of S.rautanenii(Diniz 1973).The woodlands extend northwards where they gradually change into typical miombo woodland with Julbernardia paniculata,Brachystegia bakeriana and B. spiciformis(Coelho 1964,1967;Santos 1982;Fanshawe 2010).Southwards,the tree layer becomes more open and intermingled with Acacia species(Burke 2002).Every year, about 20%of the study area burns(Stellmes et al.2013a). Most fires occur at the end of the dry season with a high intensity,causing tree damage in all diameter classes (Stellmes etal.2013a;Schelstraete 2016).
The human population in the Namibian part of the study area grew exponentially since the 1960s,fuelled by refugees of the civil war in Angola(Girot 1998;Mendelsohn 2009)and expanding along roads and dry riverbeds. Woodlands are cleared for agriculture,despite the infertile soils,resulting in a yearly deforestation rate of 3.9%for the period 1943–1996(Mendelsohn 2009).Charcoal is not produced and shifting cultivation is not practiced; farmers remain on the same fields and use fallow periods (Pröpper et al.2010).At the Angolan side,people are returning since the end of the war in 2001 and have started to clear more forest(Schneibel et al.2013).The local population harvests small quantities of wood and within a radius of about 5 km of their villages,mainly as firewood and for construction.Commercial logging focuses on large individuals ofa few species,mainly P.angolensis,and also B.plurijuga.Legal harvesting in Namibia is currently restricted to community forests,but illegal logging does take place(Kabajani 2013;Pröpper and Vollan 2013;own observations).Harvesting of timber in Angola is increasing(Karen Nott,pers.comm.,2015).
Forest inventory data
Forest inventory data were obtained from different sources:1)research data:own data collected in 217sample plots during the period 2011 – 2014 along transects representing an increasing distance from human settlements and rivers(mean distance between plots on transects:520 ± 400 m);2)forestry data:data of the Namibian Directorate of Forestry and the Namibian Community Forestry programme in 704 plots,collected by systematic grid sampling during the period 1998 – 2008 (mean distance between plots in grid:720 ± 300 m) (Chakanga and Selanniemi 1998;Kamwi 2003;Kanime 2003;Rechberger 2008);and 3)historical data of the Department of Forestry of South Africa,then in charge of forestry in Namibia,collected in 492 sample plots in a systematic grid design in the Kavango region over the period 1972 – 1974(mean distance between plots in grid:3.0 ± 2.8 km)(Geldenhuys 1992).

Table 1 Physicalenvironment and woodland characteristics in the study area as determined for the sample plots(n=1230).Climate data were derived from WorldClim(Hijmans et al.2005)and CRUTS(Harris et al.2013)
The sampling intensity of the different datasets was not the same for all stem diameter at breast height(DBH)classes.Trees with a minimum DBH of 20 cm were used for the classification to minimise differences in sampling intensities and to avoid the inclusion of early succession tree communities.The most common tree species take about 40 to 70 years from the last resprouting occurrence to reach this diameter(Worbes n.d.;Therrell et al.2007;Van Holsbeeck et al.2016).Trees with 20 cm minimum DBH were measured in plots with a radius of 20 m for the recent and 30 m for the historical dataset (Geldenhuys 1992;Burke 2002).A Monte Carlo analysis showed that the β-diversity-Whittaker’s species turn over-of the historical dataset fell within range of the research data and that the difference between the research and forestry data is higher(Additional file 1:Table S1).Structural composition of the communities could be described with data using a DBH threshold of 10 cm.Dead trees were included in the recent inventories.
The approximate locations of the historical plots were derived after georeferencing scanned forest maps of Kavango of 1971 with scale 1:75,000 used during the planning phase of the inventory.Error of the rectification varied between 9 m and 29 m,but the positional error of the sample points is larger because of limited means to determine accurate geographic coordinates in the 1970s.The historical plots were randomly clustered in groups of four and at a maximum distance of 500 m of the centralsample point.
Site variables
Climate and temperature data were obtained from WorldClim(Hijmans et al.2005)and frost days from CRUTS(Harris et al.2013).Soil data were extracted from the Harmonised World Soil Database(HWSD). Elevation data from the Shuttle Radar Topography Mission(SRTM)of NASA,were used to derive slope,aspect and landscape curvature at landscape level.The latter quantifies the shape of the landscape surface(Jenness 2006).Human intervention in the woodlands was indirectly reflected by distances to villages,tracks,roads, riverbeds,agricultural fields and major towns as human activities are concentrated there(own observations; Schneibel et al.2013).GIS data based on orthophotos of 1996 were updated in QGIS with information of Google Earth for the period 2011 to 2013.The extent of cleared land in 1972(Mendelsohn and Obeid 2004)was used to determine distances to agricultural fields for the historical dataset.Existing GIS data on groundwater depth (Bittner 2002;Christelis and Struckmeier 2011)and cattle density in Namibia was used(Mendelsohn and Obeid 2004).Tree damage caused by browsing cattle,harvesting and fire were recorded in the field for the research dataset using damage classes at plot level.Fire frequency could be determined for the recent datasets based onAVHRR for 1981–1991(Barbosa et al.1999),Landsat Quicklooks for 1991–2004(Verlinden 2004)and MODIS burned area products for 2000 – 2012(Stellmes et al. 2013a).The enhanced vegetation index(EVI)was derived from MODIS for 2000–2012(Stellmes et al.2013b).
Data analysis
Vegetation classification was tested for several abundance measures:stem density per ha,basal area per ha and presence/absence(P/A)of the tree species.The distribution of basalarea data was skewed and hence log converted to improve assumptions of normality and focus on relative quantities of species(McCune et al. 2002).Outliers and species that only occurred in three plots or less were removed.Outliers consisted of species and plots situated at more than two standard deviations from the grand mean Sørensen distance measure.For each of the abundance measures,the sample plots were hierarchically clustered into groups using a Sørensen distance measurement and flexible beta linkage(b= −0.25) (McCune and Mefford 2011).
Indicator Species Analysis was applied to determine indicator species for the different groups(Dufrene and Legendre 1997).The occurrence of single species and species combinations were evaluated based on their positive predictive value A(probability that plot belongs to community if species is present)and their sensitivity B(relative occurrence in plots of that community), which give the indicator value IV when multiplied(De Cáceres et al.,2010).Differences between groups and homogeneity within groups were tested with multiresponse permutation procedures(MRPP)using the Sørensen distance measure.The test statistic T describes the separation between the groups and the statistic A the within-group homogeneity.A is larger than 0 if the emerging groups are significantly more homogeneous than expected by chance(McCune and Mefford 1999). Communities were identified based on the results of indicator species analysis and MRPP,both performed in PC-ORD.
The impact of site variables on tree communities was explored by developing a boosted regression tree model (BRT)for each tree community.BRT models combine a large number of simple tree models and often outperform other modelling methods(Elith et al.2006,2008; Araújo and New 2007;Aertsen et al.2010).All data were used for developing the models as this gave the best predictive performance,despite the lower positional accuracy of the historical plots.A Bernoulli distribution was used to model the presence of each community. The BRT models were simplified by dropping variables that did not increase model performance.Performance of the models was evaluated by 10-fold cross-validation of deviance and correlation(Leathwick et al.2006;Elith et al.2008).Deviance measures how well calibrated the prediction values are and penalises errors in scaling of prediction values(Phillips and Dudík 2008).The effects of the predictors were studied through their contribution to the models and their partial dependence plots.The plots visualise the marginal effect of the selected variable on the model prediction,while the dependency of the remaining variables is neutralised (De’Ath 2007; Ridgeway 2014).Differences between the communities for each of the structural characteristics and site variables were tested with Kruskal-Wallis,pairwise comparisons with the Wilcoxon rank sum test and the Bonferroni adjustment for multiple tests.Certain tests could only be performed for the recent data as site variables were not available for the historical data.Spearman rank correlations were determined between all variables.BRT modelling and statistical tests were performed with the software R (Core Team R 2012),including the gbm package(Ridgeway 2014)and functions written by Elith et al.(2008).
For detection of trends in the composition of the woodland over the last 40 years,historical plots situated within a radius of 1 km of recent plots were selected.Structural composition of the historical and recent plots was compared through the total amount of stems,mean DBH and basal area for stems with minimum DBH of 10 cm,and for stems and basal area of the DBH class 10 to 20 cm.The species composition was analysed through the basal area of the most common tree species with minimum DBH of 20 cm.Differences between historical and recent data were tested for significance with the paired t-test for normal distributed data and Wilcoxon signed rank test for non-parametric data.
Results
Tree communities
The original data of all combined data inventories(n= 1413)indicated that six species contributed to 84%of the wood basal area in the study area(Additional file 2: Table S2).About 4%of the plots did not have trees with DBH of 20 cm or larger and were removed.The final dataset used for vegetation classification(n=1230)comprised 19 tree species and had a β-diversity of 5.4.Some Commiphora and Strychnos species were only included at genus level as species identification for some inventories was questionable.The abundance measure with the best classification results was log converted basal area,yielding five tree communities of which four were characterised by only one important indicator species (Table 2).Differences between the communities(A= 0.33,T= −496.4)were much larger than those between the different data sources(A=0.02,T= −38.4)or location within the study area(A=0.08,T= −76.9).

Table 2 Tree communities with indicator species and their positive predictive value(A),sensitivity(B)and indicator value (IV).Single indicator species are included when p < 0.05 and indicator species combinations when they have a higher A than the best single indicator species
The use of basal area resulted in communities with large differences in structural composition(Table 3). There was a significant,but low correlation between the density of dead trees and fire frequency for the periods 1981–1991(rs=0.11,p=0.002)and 1991–2004(rs= 0.16,p < 0.001).T.sericea was the species with the highest ratio of dead versus living trees(106%),followed by Dialium englerianum(31%)and B.africana(19%).
The BRT models for the different tree communities could explain 6 to 27%of the deviance,with the best model for the B.plurijuga community(Additional file 3: Table S3).Abiotic variables appeared to have a more important effect on tree composition than anthropogenic variables.Elevation was considered an abiotic variable as it was only correlated to climatic variables(e.g.rainfall in wettest quarter:rs= −0.77,p < 0.001)and groundwater level(rs=0.46,p < 0.001).The distance to the Okavango river was correlated to both abiotic and anthropogenic variables(e.g.burned area for 1981 – 2004(rs=0.66, p < 0.001),diurnal range(rs= −0.59,p < 0.001)).
The Kruskal-Wallis tests of site variables(Additional file 4:Table S4)indicated that three communities were typical for the sandy plateaux that are 20 to 40 m higher than the valleys:the Burkea africana,Guibourtia coleosperma and Pterocarpus angolensis-Dialium englerianum communities.The two others were found nearer to dry riverbeds,the old floodplain of the Kavango river or dune areas and are referred to as the slope communities, despite the fact that most slopes were very gradual and often invisible in the field(Fig.2d).A new BRT model was created for the distinction of sand and slope communities,which yielded a better deviance(31%)and correlation(0.61).The most important predictor of the model is cattle density(Table 4),a proxy for both abiotic variables(e.g.rainfall in the warmest quarter:rs= 0.58,p < 0.001)and disturbances(e.g.distance to villages:rs= −0.75,p < 0.001).Fire frequency was notselected as a predictor by the model.The effects of the predictors were studied through the partial dependence plots of which some are illustrated in Fig.2. Most plots on communal land belonged to the slope communities(59%),while those in the community forests were mainly sand plateau communities(83%). Stem density within the more protected state forest consisted of 41%of small stems compared to 55% on average.The BRT model information in combination with the site variables(Table S4)and structural characteristics(Table 3)were used to describe the tree communities(Additional file 5:Document S5).
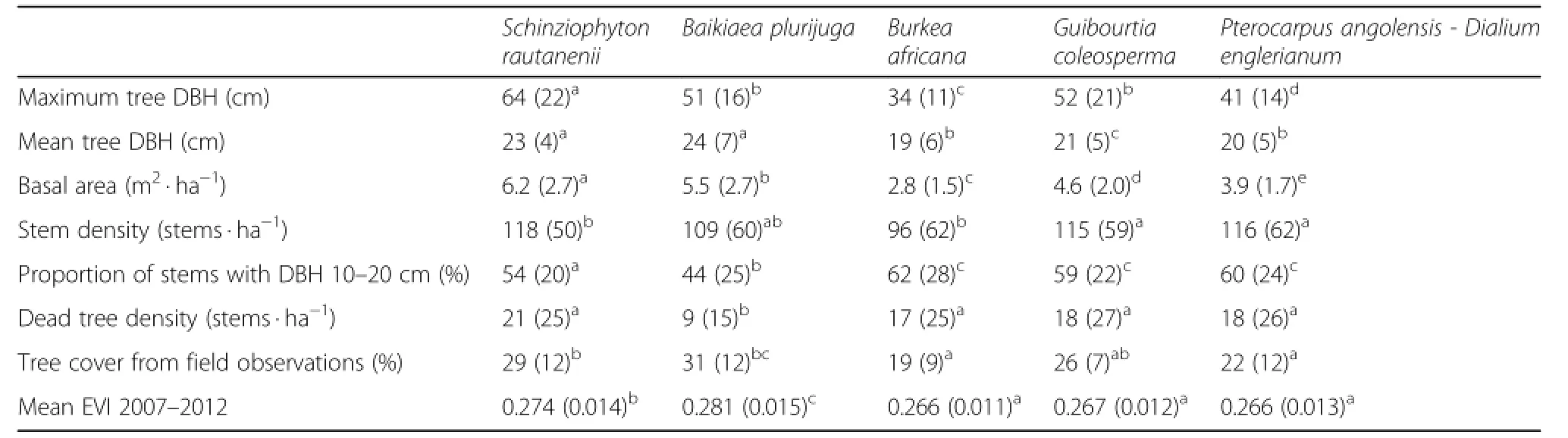
Table 3 Structuralcharacteristics of the tree communities based on trees with minimum diameter at breast height(DBH)of 10 cm

Fig.2 Partialdependence plots ofpredictorvariables for the slope and sandy plateau communities.The plots show the dependencies of thepresence models on predictors.When modelprediction for the presence of the slope community is relatively high,prediction forpresence of the sandy plateau community is low and vice versa

Table 4 Predictor variables ofthe boosted regression tree modelto distinguish sandy plateau from slope communities
Historicaltrends in tree community composition
Fifty-five historical plots were situated within a distance of 1 km from recent plots.Structural variables of late succession communities did not change significantly over the last 40 years(Fig.3).The only significant differences in composition were a decrease in the basal areasof B.africana(p < 0.05),D.englerianum(p < 0.01)and Ochna pulchra(p < 0.01).

Fig.3 Histograms showing the differences between historicaland recent plots.Change in stem density is for stems with minimum diameter at breast height(DBH)of10 cm.Change in basalarea for Burkea africana,Dialium englerianum and Ochna pulchra is forstems with minimum DBH of20 cm
Discussion
Species and structural composition of the western
Baikiaea woodlands
The western Baikiaea woodlands did not show a large variation in woody species composition and were dominated by six species that represent 84%of the total basal area(Table S2).The five tree communities were fairly equally represented(Table 2)with the B.africana community least common.B.africana was on the other hand the most represented tree species(29% of all stems)because it was often present in other communities.The community appears to be the undifferentiated phase of the woodlands.
The best represented community was P.angolensis-D.englerianum with P.angolensis,the most important timber tree in the region,the second most common species with 8 stems ha−1(19%).Other authors indicated that both P.angolensis and B.africana are common in north-eastern Namibia and there was a suggestion to name the woodlands the Burkeo-Pterocarpetea(De Raedt 1961;Strohbach and Petersen 2007).P.angolensis was often present in other communities,supporting the high degree of species interspersion reported by Graz (2006).The biggest contributors to total basal area were B.plurijuga and B.africana(Table S2).B.plurijuga isanother important timber tree,however,was not so abundant in the study area as in the Baikiaea woodlands more towards the east(Childes and Walker 1987; Mitlöhner 1993).
We are aware of the limitations of using a threshold DBH of 20 cm to perform tree community classifications.However,the species diversity(α =3)was very similar to that for the recent data with DBH threshold of 10 cm(α =3.6).The communities distinguished were also very similar to those obtained during earlier test runs performed on the recent dataset with threshold DBH of 10 cm.The main difference was the lack of a community characterised by Acacia erioloba,Terminalia sericea and Combretum spp.;a community combining an early succession vegetation with vegetation at the fringes of depressions,dry riverbeds and dune valleys (Burke 2002,2006).The main aim of the study,to distinguish long-term patterns in later succession tree communities,is therefore possible with the DBH threshold of 20 cm.Follow-up studies that focus on succession and recent dynamics are however necessary,especially with the increasing fire occurrences(Pricope and Binford 2012),ongoing deforestation,conversion to commercial cattle farms and the unknown effect of climate change.
Impact of abiotic variables on forest composition
The available information allowed creating predictive models for tree communities that could explain up to 31%of the deviance,which is in the range of other studies(Elith et al.2008;Froeschke et al.2010).The system has an inherent stochastic component because of the unpredictable rainfall events(Holdo 2006),which in turn affect the extent of the burned area(Archibald et al.2009).Overall,abiotic variables influenced the late succession tree communities more than anthropogenic induced disturbances.
The slope communities had a lower temperature seasonality and slightly higher minimum temperatures in the coldest month,suggesting that the communities are more frost sensitive than the sandy plateau communities. This explains their prevalence on slopes and northerly aspects.Other authors indicated that B.plurijuga is often found on slopes because it is frost sensitive and does not tolerate waterlogging(Childes and Walker 1987;Holdo and Timberlake 2008).The open B.africana community can be found in areas with the most extreme conditions:in areas with lowest rainfall,lower temperatures,more frost,further away from fossil rivers and often exposed to the prevailing easterly winds. There was no relation between the presence of the community and fire frequency,confirming the results of a fire trial study of Geldenhuys(1977)and that the occurrence of the B.africana community is influenced by abiotic variables.The B.africana community appears to be an earlier succession stage than the other communities with T.sericea a typical pioneer.B.africana and especially T.sericea have shallow root systems and can tolerate frost(Childes and Walker 1987;Holdo 2006). The earlier succession stage explains the higher mortality rates of T.sericea(106%)and B.africana(19%)as trees with DBH over 20 cm are relatively old and succumb to natural self-thinning.Childes and Walker (1987)also found a high mortality(63%)for T.sericea trees with DBH > 6 cm.
One of the most visible effects of climate change is tree mortality caused by warming and drought,which is globally on the increase(Allen et al.2010).The historic forest inventory did not include dead trees and so an increasing mortality trend could not be shown in our study.However,the high mortality of D.englerianum in the period 1998 – 2014 is alarming and will need further investigation.D.englerianum is valuable to the local population for its edible fruits,seeds and its wood(Mannheimer and Curtis 2009).The species has the smallest distribution of the six indicator species within the study area(Curtis and Mannheimer 2005)which coincides with a slightly lower maximum temperature of the warmest season.Warmer summers may affect this species,but another possibility is that the species cannot tolerate late dry season fires,as shown by Geldenhuys (1977).The other indicator species of the community,P. angolensis,did not show high mortality rates.However, a high mortality of P.angolensis was observed in a nearby study area with slightly lower rainfall(Strohbach and Petersen 2007)and may be a sign of the species’vulnerability to the predicted trend of decreasing summer rainfall(De Cauwer et al.2014).Monitoring tree mortality and regeneration at the edge of the transition to shrub land may show a clearer trend.This would also contribute to global predictions on the distribution of dry forests,something which is currently lacking(Liu and Yin 2013).
Impact of disturbances on forest composition
The expansion of agriculture and human infrastructure mainly threatens the slope communities.Strohbach (2013)indicated that 90%of the Acacia erioloba-S. rautanenii community in the Okavango river valley has already been cleared for agriculture.Environmental impact studies for agricultural development projects should advise preservation and establishment of corridors and islands of natural woodland,especially for the B.plurijuga and S.rautanenii communities.The slightly better soils(Table S4)and denser canopy of the slope communities allow a higher moisture retention capacity (Wallenfang et al.2015).The corridors and islands can therefore also act as refuges for certain species duringdrier conditions and be part of a climate adaptation strategy(Liu and Yin 2013).
Effects of livestock on late succession forest composition were not obvious as there was little evidence of livestock damage.The high contribution of cattle density to the predictive model can be best explained by its correlation to other variables.Areas with high cattle density are often bush encroached areas with few large trees, and were thus not included in this study.A study of the effect of livestock on woodland succession in the slope communities may give more insight in the impact of this disturbance on forest composition.
Fire frequency in the sandy plateau communities was slightly higher,causing a higher proportion of smaller stems,which is a result of frequent fires in the late dry season(Higgins et al.2007).The P.angolensis-D. englerianum community was found in the highest fire frequency areas for the period 1981–2004.The main indicator species,P.angolensis,has a high fire resistance and needs a minimum amount of fire to favour regeneration(Banda et al.2006).
The B.plurijuga community had the lowest fire frequency,indicating that the community can only evolve if fire frequency is low enough as found by other studies (Geldenhuys 1977;Childes and Walker 1987).The lower fire frequency can be explained by the occurrence of the community on slightly better soils and closest to agricultural fields,as observed by other authors(Mitlöhner 1993;Revermann and Finckh 2013;Wallenfang et al. 2015).Areas closer to agricultural fields had lower fire frequency(rs0.70,p < 0.001),which is a common occurrence(Lavorel et al.2007;Archibald et al.2009),and can thus act as a refuge for more fire sensitive species. The protection of large,mainly uninhabited areas as national parks,such as Bwabwata in Namibia,may be a disadvantage for the B.plurijuga community because of higher fire frequencies in areas further from human infrastructure.Establishment of fire refuge areas should be considered within the newly established Kavango Zambezi Transfrontier Conservation area.
Trends in forest composition over the last 40 years
Despite the human population increase of the last 40 years,no changes in the structural composition of the forest were detected for DBH classes of 10 cm and more.It may be that the totalimpact of disturbances,especially fire and harvesting of trees,remained similar as legal timber harvesting decreased after the 1980s.There were however significant changes in species composition of trees with minimum DBH of 20 cm.The decrease of O.pulchra may be explained by the fire sensitivity of the species(Geldenhuys 1977),although more evidence would be needed.The decrease in basal area of D.englerianum confirmed the high mortality rate of the species,which is probably caused by climate change but possibly by fire.The slightly decreasing basal area of B. africana over the last 40 years cannot be explained by climate change as its current range extends to areas with conditions similar to climate changes predictions(Curtis and Mannheimer 2005;De Cauwer et al.2014).A decrease in B.africana may indicate a trend towards later woodland succession stages and would need to be confirmed by revisiting more historicalplots.
Conclusions
The western Baikiaea woodlands of southern Africa are characterised by six tree species that represent most of the growing stock and can be appropriately described as Baikiaea Pterocarpus woodlands.The study was able to distinguish and describe five late successional tree communities that can serve as a benchmark for future studies focusing on the impact of climate change on forest composition.The communities were linked to small differences in the physical environment that explain their long-term resistance to disturbances,especially fire but also drought and frost events.Tree communities near valleys and slight slopes are most threated by human expansion,but are more sheltered from harsh climate conditions and fire.Their protection can be part of a climate adaptation strategy as they may act as a refuge for certain species during drier conditions.The Pterocarpus angolensis-Dialium englerianum community showed signs of a higher vulnerability to climate change.The community deserves further research focus as it may provide early warning signs of climate change and because it contains a large portion of the valuable timber tree P.angolensis. Follow-up studies are needed to study short term dynamics,especially mortality and regeneration near the edges of the transition zone between dry forest and shrub land,as is required for alltropicaldry forests.
Additional files
Additionalfile 1:Table S1.(XLS 14 kb)
Additionalfile 2:Table S2.(XLS 16 kb)
Additionalfile 3:Table S3.(XLS 20 kb)
Additionalfile 4:Table S4.(XLS 29 kb)
Additionalfile 5:Document S5(DOCX 22 kb)
Acknowledgements
We acknowledge the support and data of the Namibian Directorate of Forestry(DoF)and the Namibian Community Forestry project.The Namibian Ministry ofEnvironmentand Tourism issued the permits to perform field work.We would especially like to thank Dr.Jonathan Kamwiforhis assistance with the DoF data and Dr.Marion Stellmes for providing the EVI and burned area data derived from MODIS.We are thankfulto the team of the Universities ofGöttingen and Stellenbosch(prof.Christoph Kleinn and CoriHam),Dr.Patrick Graz,Dr.Johannes Stoffels,Rasmus Revermann,Miguel Hilario and Fransiska Kangombe for their contributions to field work.
Funding
This study was possible through supportofThe Future Okavango(TFO)and the SASSCAL projects which were funded by the German FederalMinistry of Education and Research underpromotion numbers 01 LL 0912 A and 01 LG 1201 M respectively.Bart Muys acknowledges support by the KLIMOS
ACROPOLIS research platform(Belgian Development Aid through VLIR/ARES).
Authors’contributions
VDC,CG and MK collected the data.VDC performed the data analysis.VDC, CG,RA and BM helped to draft manuscript.Allauthors read and approved the finalmanuscript.
Competing interests
The authors declare thatthey have no competing interests.
Author details
1Namibia University of Science and Technology,Faculty of Natural Resources and SpatialSciences,Private Bag 13388,Windhoek,Namibia.2University of Leuven,Division Forest,Nature and Landscape,Celestijnenlaan 200E box
2411,BE-3001 Leuven,Belgium.3Forest&Wood Science,Stellenbosch
University,c/o Forestwood cc,Murrayfield,Pretoria,South Africa.4University of Leuven,Division Ecology,Evolution and Biodiversity Conservation,
Kasteelpark Arenberg 31-2435,BE-3001 Leuven,Belgium.
Received:26 April2016 Accepted:26 August 2016
Aarrestad PA,Masunga GS,Hytteborn H etal(2011)Influence ofsoil,tree cover and large herbivores on field layer vegetation along a savanna landscape gradient in northern Botswana.JArid Environ 75:290–297.doi:10.1016/j. jaridenv.2010.10.009
Aertsen W,KintV,Van Orshoven Jetal(2010)Comparison and ranking of different modelling techniques for prediction ofsite index in Mediterranean mountain forests.Econ Model221:1119–1130
Allen CD,Macalady AK,ChenchouniH et al(2010)A globaloverview ofdrought and heat-induced tree mortality revealsemerging climate change risks for forests.For Ecol Manage 259:660–684
Araújo MB,New M(2007)Ensemble forecasting of species distributions.Trends Ecol Evol(Amst)22:42–47.doi:10.1016/j.tree.2006.09.010
Archibald S,Roy DP,Wilgen V etal(2009)Whatlimits fire?An examination of drivers ofburnt area in Southern Africa.Glob Chang Biol15:613–630
Banda T,Schwartz MW,Caro TM(2006)Effects offire on germination of Pterocarpus angolensis.For Ecol Manage 233:116–120
Barbosa PM,Stroppiana D,Grégoire J-M,Cardoso Pereira JM(1999)An assessment ofvegetation fire in Africa(1981–1991):Burned areas,burned biomass,and atmospheric emissions.Global Biogeochem Cycles 13:933–950
Barnes JI,MacGregor JJ,Nhuleipo O,Muteyauli PI(2010)The value of Namibia’s forestresources:Preliminary economic assetand flow accounts.Dev South Afr 27:159–176.doi:10.1080/03768351003740373
Bittner A(2002)Hydrogeology ofthe Kavango region.BIWAC,Windhoek
Blackie R,Baldauf C,Gautier D et al(2014)Tropicaldry forests.The state ofglobal knowledge and recommendations forfuture research.CIFOR,Bogor
Bond W,Midgley G,Woodward F(2003)What controls South African vegetationclimate or fire?S Afr JBot 69:79–91
Burke A(2002)Present vegetation ofthe Kavango.JNamibia Sci Soc 50:133–145
Burke A(2006)Savanna trees in Namibia-Factors controlling theirdistribution at the arid end ofthe spectrum.Flora-Morphol,Distrib,Funct Ecology Plants 201:189–201.doi:10.1016/j.flora.2005.06.011
CabralAIR,Vasconcelos MJ,Oom D,Sardinha R(2011)Spatialdynamics and quantification ofdeforestation in the central-plateau woodlands of Angola (1990–2009).ApplGeogr 31:1185–1193
Cauwer D(2015)Towards estimation ofgrowing stock for the timber tree Pterocarpus angolensis in Namibia.Bridging the gap between forest information needsand forestinventory capacity.University ofGoettingen, Pietermaritzburg
Chakanga M,SelanniemiT(1998)Forest Inventory report of Nkurenkuru concession area.Namibia Finland Forestry Programme,Windhoek
Chidumayo EN,Gumbo DJ(2010)The dry forests and woodlands ofAfrica: managing for products and services.Earthscan
Childes SL,Walker BH(1987)Ecology and dynamics ofthe woody vegetation on the Kalaharisands in Hwange NationalPark,Zimbabwe.Vegetatio 72:111–128
Christelis G,Struckmeier W(eds)(2011)Groundwater in Namibia.An explanation to the Hydrogeologicalmap.Second.Department of Water Affairs,Windhoek
Coelho H(1964)Contribuição para o Conhecimento da Composição Florística e Possibilidades de uma Zona Compreendida entre os rios Cubango,Cueio e Quatir.Agronomia Angolana 20:49–82
Coelho H(1967)Zonagem Florestaldo Distrito do Cuando-Cubango.Primeiros elementos.Agronomia Angolana.,pp 3–28
Core Team R(2012)R:A language and environmentforstatisticalcomputing.R Foundation for StatisticalComputing,Vienna
Curtis B,Mannheimer C(2005)Tree Atlas of Namibia.NationalBotanicalResearch Institute,Windhoek
De Cauwer V(2013)Mashare-Woody Vegetation.Biodiversity Ecol5:117.doi:10. 7809/b-e.00262
De Cauwer V,Muys B,Revermann R,Trabucco A(2014)Potential,realised,future distribution and environmentalsuitability for Pterocarpus angolensis DC in southern Africa.For EcolManage 315:211–226.doi:10.1016/j.foreco.2013.12.032
De Cáceres M,Legendre P,MorettiM(2010)Improving indicator species analysis by combining groups ofsites.Oikos 119(10):1674–1684
De Raedt JA(1961)Ontginning van inheemse bosse.Suidwes,Afrika
De’Ath G(2007)Boosted trees for ecologicalmodeling and prediction.Ecology 88:243–251
Diniz AC(1973)Características mesológicas de Angola:Descrição e correlação dosaspectos fisiográficos dos solos e da vegetação das zonas agrícolas angolanas.Missão de inquéritos agrícolas de Angola,Nova Lisboa
Dufrene M,Legendre P(1997)Species assemblages and indicatorspecies:the need for a flexible asymmetricalapproach.Ecol Monogr 67:346–366
Elith J,Graham C,Dudik M et al(2006)Novelmethods improve prediction of species’distributions from occurrence data.Ecography 29:129–151
Elith J,Leathwick JR,Hastie T(2008)A working guide to boosted regression trees.J Anim Ecol77:802–813.doi:10.1111/j.1365-2656.2008.01390.x
Fanshawe DB(2010)Vegetation descriptions of the Upper Zambezidistricts of Zambia.Edited and reissued by J.R.Timberlake and M.G.Bingham. Biodiversity Foundation for Africa,Bulawayo
FAO(2000)Globalecologicalzones
FAO(2001)GlobalForest Resources Assessment 2000.Main report.FAO,Rome
Froeschke J,Stunz GW,Wildhaber ML(2010)Environmental influences on the occurrence of coastal sharks in estuarine waters.Mar Ecol Prog Ser 407:279–292
Ganzin N,Coetzee M,Rothauge A,Fotsing J-M(2005)Rangeland resources assessmentwith satellite imagery:an operationaltoolfornationalplanning in Namibia.Geochem Int 20:33–42
Geldenhuys CJ(1977)The effect ofdifferent regimes ofannualburning on two woodland communities in Kavango.South Afr For J 103:32–42
Geldenhuys CJ(1992)Stock enumeration and management planning ofthe woodlands in Kavango,2nd edn.CSIR,South Africa
Giess W(1998)A preliminary vegetation map of Namibia,3rd revised edition. Dinteria 4:1–112
Girot PO(1998)Feasability for collaborative management schemes in forest conservation areas in Namibia.Directorate of Forestry.Namibia-Finland Forestry Program,Windhoek
Graz FP(1999)A preliminary terrain feature classification ofthe Okavango region Namibia.S Afr JSurg Geo-Inf1:123–128
Graz FP(2006)Spatialdiversity ofdry savanna woodlands.In:Hawksworth DL, BullAT(eds)Forest Diversity and Management.Springer Netherlands, Dordrecht,pp 83–97
Gröngröft A,Luther-Mosebach J,Landschreiber L,Eschenbach A(2013)Mashare-Soils.Biol Ecol5:105.doi:10.7809/b-e.00259
Hansen MC,Potapov PV,Moore R et al(2013)High-resolution globalmaps of 21st-century forest coverchange.Science 342:850–853
Harris I,Jones PD,Osborn TJ,Lister DH(2013)Updated high‐resolution grids of monthly climatic observations.The CRU TS 3.10 Dataset
Hély C,Bremond L,Alleaume S et al(2006)Sensitivity of African biomes to changesin the precipitation regime.Glob EcolBiogeogr15:258–270
Higgins SI,Bond WJ,February EC etal(2007)Effects offourdecades offire manipulation on woody vegetation structure in savanna.Ecology 88:1119–1125
Hijmans RJ,Cameron SE,Parra JL et al(2005)Very high resolution interpolated climate surfaces forgloballand areas.Int JClimatol25:1965–1978
Holdo RM(2006)Elephantherbivory,frostdamage and topkillin Kalaharisand woodland savanna trees.JVeg Sci17:509–518
Holdo RM(2007)Elephants,fire,and frost can determine community structure and composition in Kalahariwoodlands.EcolAppl17:558–568
Holdo RM,Timberlake J(2008)Rooting depth and above-ground community composition in Kalaharisand woodlands in western Zimbabwe.JTrop Ecol 24:169–176.doi:10.1017/S0266467408004835
IPCC(2007)Climate Change 2007:Impacts,Adaptation and Vulnerability:Working Group IIContribution to the Fourth Assessment Report ofthe IPCC IntergovernmentalPanelon Climate Change.Cambridge University Press, Cambridge,UK
IPCC(2014)Climate Change 2014–Impacts,Adaptation and Vulnerability: RegionalAspects.Cambridge University Press,Cambridge,UK
Jenness J(2006)Grid Tools v.1.7 extension for ArcView 3(Jenness Enterprises)
Kabajani MW(2013)The population structure of Pterocarpus angolensis(Kiaat)in response to recent harvesting in western Kavango.B.Sc.Hons,Polytechnic of Namibia,Namibia
KalwijJM,de Boer WF,Mucina L et al(2010)Tree cover and biomass increase in a southern African savanna despite growing elephant population.Ecol Appl 20:222–233
KamwiJM(2003)Woody Resources Report of Hans Kanyinga Community Forest. Directorate of Forestry,Ministry of Environment and Tourism
Kanime N(2003)Woody Resource Report of Ncamangoro Community Forest
LavorelS,Flannigan MD,Lambin EF,Scholes MC(2007)Vulnerability ofland systems to fire:Interactions among humans,climate,the atmosphere,and ecosystems.Mitig Adapt Strateg Glob Chang 12:33–53
Leadley P(2010)Biodiversity Scenarios:Projections of21st Century Change in Biodiversity,and Associated Ecosystem Services:a TechnicalReport forthe GlobalBiodiversity Outlook 3.Secretariat of the Convention on Biological Diversity,Montreal
Leathwick JR,Elith J,Francis MP et al(2006)Variation in demersalfish species richness in the oceans surrounding New Zealand:an analysis using boosted regression trees.Mar EcolProg Ser321:267–281
Liu H,Yin Y(2013)Response offorest distribution to past climate change:An insight into future predictions.Chin Sci Bull58:4426–4436
Liu G,Liu H,Yin Y(2013)Globalpatterns of NDVI-indicated vegetation extremes and their sensitivity to climate extremes.Environ Res Lett 8:25009
Loarie SR,van Aarde RJ,Pimm SL(2009)Elephantseasonalvegetation preferences across dry and wet savannas.Biol Conserv 142:3099–3107
Lucht W,SchaphoffS,Erbrecht T etal(2006)Terrestrialvegetation redistribution and carbon balance under climate change.Carbon Balance Manage 1:6
Makhabu SW,Skarpe C,Hytteborn H,Mpofu ZD(2006)The plantvigour hypothesis revisited-how is browsing by ungulates and elephant related to woody species growth rate?Plant Ecol184:163–172
Mannheimer CA,Curtis BA(eds)(2009)Le Roux and Müller’s Field Guide to the Trees and Shrubs of Namibia.Macmillan Education Namibia,Windhoek
McCune B,Mefford MJ(1999)PC-ORD.Multivariate analysis ofecologicaldata. MjM Software Design,Oregon
McCune B,Mefford MJ(2011)PC-ORD.Multivariate Analysis of Ecological Data, Version 6.MjM Software Design,Oregon
McCune B,Grace JB,Urban DL(2002)Analysis of ecologicalcommunities.MjM Software Design,Oregon
Mendelsohn J(2009)Land use in Kavango:past,present and future.OKACOM, Maun
Mendelsohn J,Obeid SE(2004)Okavango River:The Flow of a Lifeline.Struik Miles L,Newton AC,DeFries RS etal(2006)A globaloverview ofthe conservation status oftropicaldry forests.J Biogeography 33:491–505
Mitlöhner R(1993)Regengrüne Baikiaea-Trockenwälderon Ost-Caprivi,Namibia. Forstarchiv 64:264–274
Mosugelo DK,Moe SR,Ringrose S,Nellemann C(2002)Vegetation changes during a 36-yearperiod in northern Chobe NationalPark,Botswana.Afr J Ecol40:232–240.doi:10.1046/j.1365-2028.2002.00361.x
Phillips SJ,Dudík M(2008)Modeling ofspecies distributions with Maxent:new extensions and a comprehensive evaluation.Ecography 31:161–175.doi:10. 1111/j.0906-7590.2008.5203.x
Pricope NG,Binford MW(2012)A spatio-temporalanalysis offire recurrence and extent for semi-arid savanna ecosystems in southern Africa using moderateresolution satellite imagery.JEnviron Manage 100:72–85.doi:10.1016/j. jenvman.2012.01.024
Pröpper M,Vollan B(2013)Beyond Awareness and Self-Governance:Approaching Kavango Timber Users’Real-Life Choices.Land 2:392–418.doi:10.3390/ land2030392
Pröpper M,Gröngröft A,Falk T,etal.(2010)Causes and perspectives ofland-cover change through expanding cultivation in Kavango,Gottingen&Windhoek
Rechberger S(2008)Likwaterera community forest.Participatory Natural Resource Assessment(PNRA).Community Forestry in north-eastern Namibia, Windhoek
Revermann R,Finckh M(2013)Caiundo-Vegetation.Biodiversity Ecol5:91–96. doi:10.7809/b-e.00255
Ridgeway G(2014)Package “gbm”.Generalized Boosted Regression Models
Rogelj J,Meinshausen M,Knutti R(2012)Globalwarming under old and new scenarios using IPCC climate sensitivity range estimates.Nature Climate Change 2(4):248–253
Sankaran M,Hanan NP,Scholes RJetal(2005)Determinants ofwoody coverin African savannas.Nature 438:846–849.doi:10.1038/nature04070
Sankaran M,Ratnam J,Hanan N(2008)Woody cover in African savannas:the role ofresources,fire and herbivory.Glob EcolBiogeogr 17:236–245
Santos RM(1982)Itinerários florísticos e carta da vegetação do Cuando-Cubango. Instituto de Investigação Científica Tropical,Lisbon
Schelstraete M(2016)Assessment offire damage on the forest population near Hamoye,Kavango.M.Sc.Thesis,University of Ghent,Namibia
SchneibelA,Stellmes M,Revermann R etal(2013)Agriculturalexpansion during the post-civilwar period in southern Angola based on bi-temporalLandsat data.Biodiversity Ecol5:311.doi:10.7809/b-e.00285
Shackleton CM,Shackleton SE,Buiten E,Bird N(2007)The importance ofdry woodlands and forests in rurallivelihoods and poverty alleviation in South Africa.Forest Policy Econ 9:558–577
Sloan S,Sayer JA(2015)Forest Resources Assessment of 2015 shows positive global trends but forest loss and degradation persist in poor tropical countries.For Ecol Manage 352:134–145.doi:10.1016/j.foreco. 2015.06.013
Snyder PK,Delire C,Foley JA(2004)Evaluating the influence ofdifferent vegetation biomes on the globalclimate.Climate Dynam 23:279–302
Stellmes M,Frantz D,Finckh M,Revermann R(2013a)Fire frequency,fire seasonality and fire intensity within the Okavango region derived from MODIS fire products.Biodiversity Ecol5:351.doi:10.7809/b-e.00288
Stellmes M,Frantz D,Finckh M,Revermann R(2013b)Okavango Basin-Earth Observation.Biodiversity Ecol5:23.doi:10.7809/b-e.00239
Strohbach(2013)Vegetation ofthe Okavango River valley in Kavango West, Namibia.Biodivers Ecol5:321–339
Strohbach BJ,Petersen A(2007)Vegetation of the centralKavango woodlands in Namibia:An example from the Mile 46 Livestock Development Centre.S Afr JBot73:391–401
Therrell MD,Stahle DW,MukelabaiMM,Shugart HH(2007)Age,and radial growth dynamics of Pterocarpus angolensis in southern Africa.For Ecol Manage 244:24–31
Thijs KW,Aerts R,Musila W etal(2014)Potentialtree species extinction,colonization and recruitment in Afromontane forestrelicts.Basic ApplEcol15:288–296
Thuiller W,Midgley GF,Hughes GO et al(2006)Endemic species and ecosystem sensitivity to climate change in Namibia.Glob Chang Biol 12:759–776
Van Holsbeeck S,De Cauwer V,De Ridder M et al(2016)Annualdiameter growth of Pterocarpus angolensis(Kiaat)and other woodland species in Namibia.For EcolManag 373:1–8.doi:10.1016/j.foreco.2016.04.031
Verlinden A(2004)Number oftimes different places burnt during the past 13 years,Kavango.In:Okavango River:The flow ofa lifeline.NationalRemote Sensing Centre Namibia,Windhoek,
Wallenfang J,Finckh M,Oldeland J,Revermann R(2015)Impactofshifting cultivation on dense tropicalwoodlands in southeast Angola.Tropical Conservation Science 8:863–892
Worbes M(n.d.)Growth oftrees from Namibia.A dendrochronologicalstudy. Unpublished report
WWF(2012)Miombo Eco-region.Home ofthe Zambezi.Conservation strategy 2011–2020.WWF,Harare
Zhu K,Woodall CW,Ghosh S et al(2014)Dualimpacts ofclimate change:forest migration and turnoverthrough life history.Glob Chang Biol20:251–264
*Correspondence:vdecauwer@nust.na
1Namibia University of Science and Technology,Faculty of NaturalResources and Spatial Sciences,Private Bag 13388,Windhoek,Namibia
2University ofLeuven,Division Forest,Nature and Landscape,Celestijnenlaan 200E box 2411,BE-3001 Leuven,Belgium
Fulllistofauthorinformation is available atthe end ofthe article
Methods:This study combined historicaland modern forest inventories covering an area of21,000 km2in a transition zone in Namibia and Angola to distinguish late succession tree communities,to understand their dependence on site factors,and to detect trends in the forest composition over the last 40 years.
Results:The woodlands were dominated by six tree species that represented 84%ofthe totalbasalarea and can be referred to as Baikiaea-Pterocarpus woodlands.A boosted regression tree analysis revealed that late succession tree communities are primarily determined by climate and topography.The Schinziophyton rautanenii and Baikiaea plurijuga communities are common on slightly inclined dune or valley slopes and had the highest basalarea (5.5 – 6.2 m2ha−1).The Burkea africana-Guibourtia coleosperma and Pterocarpus angolensis – Dialium englerianum communities are typicalfor the sandy plateaux and have a higher proportion of smaller stems caused by a higher fire frequency.A decrease in overallbasalarea or a trend of increasing domination by the more drought and cold resilient B.africana community was not confirmed by the historicaldata,but there were significant decreases in basalarea for Ochna pulchra and the valuable fruit tree D.englerianum.
Conclusions:The slope communities are more sheltered from fire,frost and drought but are more susceptible to human expansion.The community with the important timber tree P.angolensis can best withstand high fire frequency but shows signs ofa higher vulnerability to climate change.Conservation and climate adaptation strategies should include protection ofthe slope communities through refuges.Follow-up studies are needed on short term dynamics,especially near the edges of the transition zone towards shrub land.
- Forest Ecosystems的其它文章
- Romanian legalmanagement rules limit wood production in Norway spruce and beech forests
- Life cycle cost and economic assessment of biochar-based bioenergy production and biochar land application in Northwestern Ontario,Canada
- Adaptation of forest management to climate change as perceived by forest owners and managers in Belgium
- Drought sensitivity ofbeech on a shallow chalk soilin northeastern Germany – a comparative study
- Structure and dendroecology of Thuja occidentalis in disjunct stands south ofits contiguous range in the central Appalachian Mountains,USA
- Development and evaluation ofan individualtree growth and yield modelfor the mixed species forest ofthe Adirondacks Region of New York,USA

