湖泊沉积物中磷酸盐氧同位素前处理方法对比*
张秀梅,王亚蕊,马书占,陈向超,冯慕华
(1:中国科学院南京地理与湖泊研究所湖泊与环境国家重点实验室,南京 210008)(2:中国科学院大学,北京 100049)(3:中国科学院大学中丹学院,北京 100190)(4:苏州科技大学环境科学与工程学院,苏州 215011)
湖泊沉积物中磷酸盐氧同位素前处理方法对比*
张秀梅1,2,3,王亚蕊1,2,马书占1,4,陈向超1,2,冯慕华1**
(1:中国科学院南京地理与湖泊研究所湖泊与环境国家重点实验室,南京 210008)(2:中国科学院大学,北京 100049)(3:中国科学院大学中丹学院,北京 100190)(4:苏州科技大学环境科学与工程学院,苏州 215011)
磷酸盐氧同位素(δ18OP)是一种有效的磷源示踪方式. 由于湖泊沉积物的组成十分复杂,必须对样品进行除碳处理和纯化处理. 本文对目前应用较为广泛的几种前处理方法进行比较,包括NaClO处理和H2O2处理等除有机碳方法,以及Blake法和McLaughlin法等纯化方法,以期获得适用于湖泊沉积物磷酸盐氧同位素的前处理方法. 结果表明:(1)NaClO处理可以保证较高的无机磷提取效率,同时能有效地减少无机磷提取液中有机质含量,且对不同形态磷的破坏较小;H2O2对有机质的去除效果不稳定,且处理后样品磷形态之间发生转化,显著增加了提取的无机磷浓度. (2)Blake法和McLaughlin法分别采用磷钼酸铵(APM)+磷酸铵镁(MAP)沉淀和CePO4沉淀对样品进行纯化. 在纯化处理湖泊沉积物过程中,Blake法优于McLaughlin法,主要体现为有机质去除率高,并且磷的回收率较为稳定. (3)经Blake法纯化丹麦Nordborg湖沉积物样品得到的Ag3PO4中C、N含量低于McLaughlin法且重现性好. 本文结合NaClO法与Blake法的优点,建立了一种适合湖泊沉积物的磷酸盐氧同位素前处理方法:首先用2.5% NaClO对沉积物样品进行除碳预处理,然后对磷酸盐提取液依次通过氢氧化镁共沉淀(MAGIC)、APM+MAP沉淀、阳离子交换树脂处理,最后生成Ag3PO4沉淀.
沉积物;磷酸盐氧同位素;前处理;除有机碳;无机磷
磷循环属于典型的沉积型循环[1].沉积物作为湖泊磷的蓄积库,汇集了各种类型的陆源磷,但在一定条件下,沉积物又由磷的“汇”转变为“源”,不断地释放到上覆水中[2-3]. 因此,探究沉积物中磷的来源,对控制和治理湖泊富营养化具有重要的指导意义.

为了探明NaClO处理和H2O2处理等除有机碳方法,Blake法和McLaughlin法等纯化方法在湖泊沉积物磷酸盐氧同位素前处理中的效果,本文对这几种方法进行了对比研究. 首先,采用2.5% NaClO和30% H2O2对有机质含量较高的湖泊沉积物样品进行预处理,并比较2种预处理对无机磷提取效率、提取液中溶解性有机碳(DOC)含量以及沉积物磷形态的影响. 其次,运用Blake法和McLaughlin法对沉积物提取液中磷酸盐进行纯化,比较2种方法中DOC的去除效率和磷酸盐的回收率. 本文结合预处理除碳方法和2种纯化方法的优点,并对一些操作步骤进行筛选优化,建立一种适用于湖泊沉积物磷酸盐氧同位素的前处理方法.
1 材料与方法
1.1 试剂与仪器
试剂:NaClO(Aladdin)、 H2O2(AR)、HCl(GR)、Mg(NO3)2·6H2O(AR)、NaOH(GR)、HNO3(GR)、(NH4)6Mo7O24·4H2O(AR)、CeNO3(Aladdin)、阳离子交换树脂(BioRad AG-50X8,H+型,100~200目)、阴离子交换树脂(BioRad AG1-X8,OH-型,200~400目)、NaHCO3(Aladdin)、MgCl2(AR)、BD试剂、CH3COOK·3H2O(GR)、CH3COOH(AR).
器皿及仪器: 聚四氟乙烯(PTFE)离心管、100 ml烧杯、1 L烧杯、10 ml玻璃离心管、恒温振荡器(HZ-9511K)、冷冻干燥机(7948030,Labconco)、离心机(TGL-16M,湘仪)、马弗炉(KBF1700)、带砂芯的层析柱、总有机碳分析仪(Torch,Teledyne Tekmar)、元素分析仪(EA3000,EuroVector).
1.2 样品采集
丹麦富营养化湖泊Nordborg湖平均水深5 m,湖面面积0.546 km2,换水周期为0.82 a[16]. 流域面积63%为农田,25%为城镇用地,其他区域被森林覆盖. 湖北岸设有两个前置库,拦截净化农田来水. 于2014年3月12日用彼得森采泥器进行表层沉积物样品采集. 4个样品分别位于Nordborg湖最深处(8.5 m)、1/2水深处(4.2 m)、前置库KS1和前置库KS2,记为样品1、样品2、样品3和样品4. 4个沉积物样品的碳、氮、磷含量如表1所示.

表1 丹麦Nordborg湖沉积物样品的碳、氮、磷含量
1.3 实验方法
1.3.1 预处理方法 将沉积物样品冻干后研磨过200目筛. 分别取0.5 g左右样品于PTFE离心管中,设置3个实验组:对照组、NaClO处理组和H2O2处理组. 每组处理3个平行. 具体步骤如下:
对照组:不进行任何预处理.
NaClO处理组:加入10 ml 2.5% NaClO溶液震荡24 h后离心(7000转/min,10 min),弃去上清液,剩余残渣用纯水洗涤4~5次至中性.
H2O2处理组:加入10 ml 30% H2O2溶液浸泡24 h后,待无明显气泡生成后离心(7000转/min,10 min),弃去上清液,剩余残渣用纯水洗涤4~5次至中性.

1.3.2 纯化方法 以样品3为例,分别按照Blake法和McLaughlin法对沉积物提取液进行纯化处理,并测定每一步的DOC浓度和磷含量,最后测定产物Ag3PO4的C、N含量. 每组处理7个平行.


1.4 数据统计与分析
采用Origin 9.0软件进行图形绘制,并用SPSS 20.0软件对数据进行统计分析.
2 结果与讨论
2.1 不同预处理方法的比较
2.1.1 不同预处理方法对DOC去除效果的影响 经NaClO处理后无机磷提取液中DOC浓度减少了32%~74%;H2O2对DOC的去除效果不稳定,经H2O2处理后样品1和样品4的无机磷提取液中DOC浓度分别减少了10%和55%,但是样品2和样品3的DOC浓度却分别增加了21%和70%. 对比2种预处理方法对样品DOC的去除结果发现,两者差异显著(P=0.01),且NaClO处理对DOC去除效果优于H2O2(图1a). 这与土壤方面的相关研究结果一致,在去除土壤有机质时NaClO比H2O2更有效[20-23].
NaClO去除天然有机质时,ClO-直接与腐殖酸、富里酸、芳香族化合物等发生氯化反应,生成CHCl3、氯代乙酸等[24-26]. 而且NaClO溶液呈碱性,可将部分腐殖酸和富里酸洗脱出来,从而减少无机磷提取液中DOC浓度.
H2O2稳定性较差,可被沉积物中的Fe3+和锰氧化物催化分解[27],从而减弱对有机质的降解作用[28]. 腐殖质、木质素以及一些简单的碳水化合物与H2O2反应会生成大量小分子有机酸(如蚁酸、乙酸、乙二酸、丙二酸)、苯酚、苯甲酸[29]. 样品2和样品3的无机磷提取液中DOC浓度升高可能是因为H2O2将沉积物样品中部分难降解有机质转化成水溶性有机酸.
2.1.2 不同预处理方法对沉积物磷形态的影响 NaClO对沉积物磷形态的影响小于H2O2. 经NaClO处理后,无机磷提取率维持在93%~98%(图1b),但沉积物的总磷减少14%~29%(图2),表明NaClO对有机磷有明显的去除效果而对无机磷几乎无影响. 经H2O2处理后,除样品1总磷减少30%外,其他3种样品的总磷与对照组几乎没有差异(图2),但无机磷的提取率提高了35%~64%(图1b),表明H2O2将有机磷转化为无机磷.

图1 2.5% NaClO和30% H2O2预处理后沉积物提取液中DOC浓度(a)和无机磷含量(b)的变化
比较NaClO和H2O2处理后沉积物磷赋存形态的变化可得,NaClO和H2O2处理组的Ex-P分别减少了29%~86%和86%~90%,说明Ex-P更易受H2O2破坏(P=0.039). Ex-P一般通过物理吸附作用或者磷酸盐与沉积相胶体、矿物通过配位交换的方式吸附在沉积物表面[30],极易因环境的改变而释放. 实验过程中,NaClO 与沉积物反应缓和,而H2O2与沉积物反应时却有大量气泡冒出,激烈的气泡涌动可能促进了Ex-P的释放. 但由于Ex-P仅占总磷的1%~3%,因此Ex-P的变化对无机磷提取率的影响可忽略.

NaClO对CFAP和CAP影响不大,含量波动范围分别为-9%~6%和-4%~3%. CFAP和CAP主要是CaCO3结合态的磷,稳定性较强,不易释放[3]. H2O2使这2种磷形态的磷含量明显增加,其中CFAP增加了21%~65%;CAP除样品3变化较小,仅减少了4%外,其它样品增加了32%~182%,其原因可能是H2O2在氧化有机质的过程中将有机磷转化为无机磷.
与对照组相比,经过NaClO和H2O2处理后Org-P含量分别减少了47%~69%和82%~96%. 这表明NaClO和H2O2均对Org-P有明显影响,且H2O2处理对Org-P影响更明显(P=0.020).
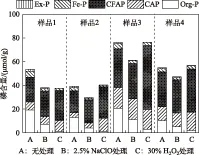
图2 2.5% NaClO和30% H2O2预处理后沉积物磷形态的变化
2.2 两种经典方法的纯化效率对比

Blake法和McLaughlin法分别采用2次MAGIC和1次MAGIC操作,DOC浓度分别减少了42.03%和33.98%,二者相差8.04%,表明第2次MAGIC操作对DOC的去除作用有限. 这可能与Mg(OH)2沉淀对有机质的吸附作用有关,在2次MAGIC操作中Mg(OH)2沉淀量基本不变,故吸附的有机质含量变化不大,减少的部分可能是被NaOH清洗掉的碱溶性有机质. 由于MAGIC操作复杂,且耗时长,因此,建议只采用1次MAGIC.
在利用特征沉淀对样品进行纯化的步骤中,Blake法与McLaughlin法分别采用了APM+MAP沉淀和CePO4沉淀,但是二者对有机质的去除效果有显著差异(P=0.007). 经APM+MAP沉淀处理后,样品中DOC浓度减少至7.74±1.13 mg/L,约为提取液DOC浓度的12.70%;而CePO4沉淀却使DOC浓度升高至提取液的2倍. Blake法中APM和MAP沉淀分别选用5% NH4NO3和1∶20氨水进行洗涤,2种试剂可分别去除酸溶性有机质和碱溶性有机质,从而使DOC浓度下降. McLaughlin法中DOC浓度升高的原因可能是在第一步中阳离子交换树脂用量不足,导致金属离子无法完全去除,在CePO4沉淀的过程中,生成金属氢氧化物. 在洗涤沉淀的过程中,大量CH3COOK会吸附于沉淀,且固液分离时有少量CH3COOK溶液仍滞留在离心管内,导致DOC浓度升高.

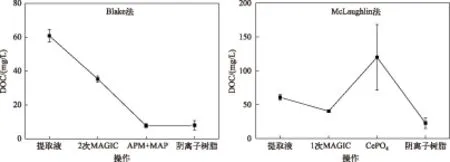
图3 Blake法和McLaughlin法对有机质去除效率对比


表2 各步骤磷回收率比较
2.3 两种经典方法的纯化效果比较
按照Blake法,将纯化后的溶液蒸发浓缩,加入银氨溶液后加热使氨水挥发析出Ag3PO4,亮黄色的结晶浮于溶液表面. 按照McLaughlin法,调节pH至7~8,加入AgNO3溶液,迅速生成黄白色的Ag3PO4沉淀,沉淀随时间慢慢变暗.
为了比较Ag3PO4纯度,分别对2种方法得到的Ag3PO4进行C、N含量测定(图4). 其中1~7和8~13分别代表用Blake法和McLaughlin法纯化样品3得到的Ag3PO4平行样. 经Blake法纯化获得的Ag3PO4的C、N含量分别为0.622%±0.081%和0.032%±0.006%;经McLaughlin法纯化获得的Ag3PO4的C、N含量分别为3.37%±0.82%和0.55%±0.21%,分别是Blake法的5.4和17.2倍,说明Blake法的纯化效果更好,重现性高,效果稳定. 这与Tamburini等[15]的研究结果一致. Tamburini等在分析不同有机质含量的土壤样品的δ18OP时发现,通过McLaughlin法纯化获得的Ag3PO4中含有有机质,且对后续的氧同位素测定有干扰;慢沉淀可以减少有机质的混入和对δ18OP测定的影响[15].

图4 经Blake法和McLaughlin法获得的Ag3PO4的C、N含量对比

图5 湖泊沉积物磷酸盐氧同位素前处理流程图
3 结论
1)NaClO处理可以保证较高的无机磷提取效率,同时能有效地减少无机磷提取液中有机质含量,且对不同形态磷的破坏较小;H2O2对有机质的去除效果不稳定,处理后沉积物的磷形态之间发生转化. 因此,NaClO 法更适用于湖泊沉积物样品的预处理.
2) Blake法对有机质的去除率高于McLaughlin法;2种方法的磷回收率差异不大,但Blake法稳定性较好. Blake法和McLaughlin法得到的Ag3PO4中C、N含量的对比分析表明,Blake法的纯化效果更好且更稳定.
3)结合NaClO法与Blake法的优点,建立了一种适合湖泊沉积物的磷酸盐氧同位素前处理方法(图5). 首先用2.5% NaClO对沉积物样品进行除碳预处理,然后对磷酸盐提取液依次通过氢氧化镁共沉淀(MAGIC)、APM+MAP沉淀、阳离子交换树脂处理,最后生成Ag3PO4.
[1] Li Bo ed. Ecology. Beijing:Higher Education Press, 2000: 251-252. [李博. 生态学. 北京:高等教育出版社,2000: 251-252.]
[2] Fan Chengxin. Physiochemical characteristics of sedimets in Gehu Lake and simulation of its phosphorus release.JLakeSci, 1995, 7(4): 341-349. DOI:10.18307/1995.0408.[范成新. 滆湖沉积物理化特征及磷释放模拟. 湖泊科学, 1995, 7(4): 341-349.]
[3] Song Yuanyuan, Feng Muhua, Su Zhengguangetal.Vertical distribution of chemical speciation of phosphorus in sediments from different sources of Fuxian Lake.ActaScientiaeCircumstantiae, 2013, 33(9): 2579-2589. [宋媛媛,冯慕华,苏争光等. 抚仙湖不同来源沉积物磷形态垂向分布特征. 环境科学学报,2013, 33(9): 2579-2589.]
[4] Davies CL, Surridge BW, Gooddy DC. Phosphate oxygen isotopes within aquatic ecosystems: Global data synthesis and future research priorities.ScienceoftheTotalEnvironment, 2014, 496: 563-575.
[5] Liang Y, Blake R. Oxygen isotope signature of Pi regeneration from organic compounds by phosphomonoesterases and photooxidation.GeochimicaetCosmochimicaActa, 2006, 70(15): 3957-3969.
[6] Mclaughlin K, Paytan A, Kendall Cetal.Phosphate oxygen isotopes as a tracer for sources and cycling of phosphate in San Francisco Bay.JournalofGeophysicalResearch, 2006, 111, G03003.
[7] Jaisi DP, Blake RE. Tracing sources and cycling of phosphorus in Peru Margin sediments using oxygen isotopes in authigenic and detrital phosphates.GeochimicaetCosmochimicaActa, 2010, 74(11): 3199-3212.
[8] Goldhammer T, Brunner B, Bernasconi SMetal.Phosphate oxygen isotopes: Insights into sedimentary phosphorus cycling from the Benguela upwelling system.GeochimicaetCosmochimicaActa, 2011, 75(13): 3741-3756.
[9] Stephan E. Oxygen isotope analysis of animal bone phosphate: Method refinement, influence of consolidants, and reconstruction of palaeotemperatures for holocene sites.JournalofArchaeologicalScience, 2000, 27: 523-535.
[10] O’Neil JR, Roe LJ, Reinhard Eetal. A rapid and precise method of oxygen isotope analysis of biogenic phosphate.IsraelJournalofEarthSciences, 1994, 43(3/4): 203-212.
[11] Blake RE, Chang SJ, Lepland A. Phosphate oxygen isotopic evidence for a temperate and biologically active Archaean ocean.Nature, 2010, 464(7291): 1029-1032.
[12] Mclaughlin K, Silva S, Kendall Cetal. A precise method for the analysis of δ18O of dissolved inorganic phosphate in seawater.LimnologyandOceanography:Methods, 2004, 2(7): 202-212.
[13] Lu Yangyang, Zheng Zhenzhen, Yin Xijieetal. The measurement of oxygen isotope composition of dissolved inorganic phosphate in seawater.ActaGeoscienticaSinica, 2012, 33(6): 961-966. [卢阳阳,郑珍珍,尹希杰等. 海水溶解磷酸盐氧同位素组成的测定. 地球学报, 2012, 33(6): 961-966.]
[14] Mclaughlin K, Cade-Menun BJ, Paytan A. The oxygen isotopic composition of phosphate in Elkhorn Slough, California: A tracer for phosphate sources.Estuarine,CoastalandShelfScience, 2006, 70(3): 499-506.
[15] Tamburini F, Bernasconi S, Angert Aetal. A method for the analysis of the δ18O of inorganic phosphate extracted from soils with HCl.EuropeanJournalofSoilScience, 2010, 61(6): 1025-1032.
[16] Egemose S, De Vicente I, Reitzel Ketal. Changed cycling of P, N, Si, and DOC in Danish Lake Nordborg after aluminum treatment.CanadianJournalofFisheriesandAquaticSciences, 2011, 68(5): 842-856.
[17] Zhang JZ, Guo L, Fischer CJ. Abundance and chemical speciation of phosphorus in sediments of the Mackenzie River Delta, the Chukchi Sea and the Bering Sea: Importance of detrital apatite.AquaticGeochemistry, 2010, 16(3): 353-371.
[18] Editorial Board of Water and Wastewater Monitoring and Analysis Methods,Ministry of Environmental Protection of the People’s Republic of China ed. Water and wastewater monitoring and analysis methods: 4th edition. Beijing: China Environmental Science Press, 2002. [国家环境保护总局《水和废水监测分析方法》编委会. 水和废水监测分析方法:第4版. 北京: 中国环境科学出版社, 2002.]
[19] Blake RE, Chang SJ, Lepland A. Phosphate oxygen isotopic evidence for a temperate and biologically active Archaean ocean.Nature, 2010, 464(7291): 1029-1032.
[20] Lavkulich LM, Wiens JH. Comparison of organic matter destruction by hydrogen peroxide and sodium hypochlorite and its effects on selected mineral constituents.SoilScienceSocietyofAmericaJournal,1970, 34: 755-758.
[21] Mikutta R,Kleber M, Kaiser Ketal. Review: Organic matter removal from soil using hydrogen peroxide, sodium hypochlorite, and disodium peroxodisulfate.SoilScienceSocietyofAmericaJournal, 2005, 69:121-135.
[22] Siregar A, Kleber M, Mikutta Retal.Sodium hypochlorite oxidation reduces soil organic matter concentrations without affecting inorganic soil constituents.EuropeanJournalofSoilScience, 2005, 56(4): 481-490.
[23] Anderson J. An improved pretreatment for mineralogical analysis of samples containing organic matter.ClaysandClayMinerals, 1963, 10(3): 380-388.
[24] Jimenez MCS, Dominguez AP, Silverio JMC. Reaction kinetics of humic acid with sodium hypochlorite.WaterResearch, 1993, 27(5): 815-820.
[25] Li CW, Benjamin MM, Korshin GV. Use of UV spectroscopy to characterize the reaction between NOM and free chlorine.EnvironmentalScience&Technology, 2000, 34(12): 2570-2575.
[26] Pomes ML, Larive CK, Thurman EMetal. Sources and haloacetic acid/trihalomethane formation potentials of aquatic humic substances in the Wakarusa River and Clinton Lake near Lawrence, Kansas.EnvironmentalScience&Technology, 2000, 34(20): 4278-4286.
[27] Strukul G. Catalytic oxidations with hydrogen peroxide as oxidant. Springer Science & Business Media. Dordrecht: Kluwer Academic Publisher, 1992: 97-98.
[28] Wang GS, Liao CH, Wu FJ. Photodegradation of humic acids in the presence of hydrogen peroxide.Chemosphere, 2001, 42(4): 379-387.
[29] Chakrabartty S, Kretschmer H, Cherwonka S. Hypohalite oxidation of humic acids.SoilScience, 1974, 117(6): 318-322.
[30] Yin Ran, Wang Fushun, Mei Hangyuanetal.Distribution of phosphorus forms in the sediments of cascade reservoirs with different trophic states in Wujiang catchment.ChineseJournalofEcology, 2010, 29(1): 91-97.[尹然, 汪福顺, 梅航远等. 乌江流域不同营养水平的梯级水库沉积物中磷形态特征. 生态学杂志, 2010, 29(1): 91-97.]
[31] Pan B, Wu J, Pan Betal. Development of polymer-based nanosized hydrated ferric oxides (HFOs) for enhanced phosphate removal from waste effluents.WaterResearch, 2009, 43(17): 4421-4429.
Comparison of pretreatment methods of oxygen isotope composition of phosphate in lake sediments
Zhang Xiumei1,2,3,Wang Yarui1,2, Ma Shuzhan1,4, Chen Xiangchao1,2& Feng Muhua1**
(1:StateKeyLaboratoryofLakeScienceandEnvironment,NanjingInstituteofGeographyandLimnology,ChineseAcademyofSciences,Nanjing210008,P.R.China)(2:UniversityofChineseAcademyofSciences,Beijing100049,P.R.China)(3:Sino-DanishCenterforEducationandResearch,Beijing100190,P.R.China)(4:SchoolofEnvironmentalScienceandEngineering,SuzhouUniversityofScienceandTechnology,Suzhou215011,P.R.China)
Oxygen isotope of phosphate (δ18OP) was a very effective proxy for tracing sources. Due to the complicated composition of lake sediments, samples must be treated by carbon elimination and purified before the δ18OPmeasurement. In order to establish a suitable pretreatment method for δ18OPof lake sediments, this study compared several widely-used pretreatment methods, including organic carbon elimination by NaClO treatment and H2O2treatment, purification by Blake method and McLaughlin method. The results showed: (1) NaClO kept high inorganic phosphate extract ratios, and removed organic matter efficiently with less impacts on different phosphate species. The organic removal efficiency by H2O2was not stable while the phosphate species changed after treatments and inorganic phosphate concentration increased significantly. (2)Ammonium phosphomolybdate(APM)+ ammonium phosphate(MAP)and CePO4was used by Blake method and McLaughlin method,respectively. Blake method had better performance on the purification of lake sediments samples than McLaughlin method, and a higher organic matter removal rate than McLaughlin method with stable phosphate throughput. (3) The Ag3PO4produced by the Blake method had lower C, N contents and better repeatability than the McLaughlin method. The advantages of both the NaClO treatment and the Blake method were made full use to establish an improved δ18OPpretreatment method for lake sediments with modified procedures. It is recommended to use 2.5% NaClO to remove organic matter before phosphate extraction, and then purify the phosphate extractions through MAGIC, APM+MAP, and cation resin treatment, in turn.
Sediments; oxygen isotope of phosphate; pretreatment method; organic carbon elimination; inorganic phosphate
*国家自然科学基金项目(41471075,41171366)资助.2016-04-19收稿;2016-08-11收修改稿. 张秀梅(1989~),女,硕士研究生;E-mail: zhxm_66@yeah.net.
J.LakeSci.(湖泊科学), 2017, 29(2): 512-520
DOI 10.18307/2017.0227
©2017 byJournalofLakeSciences
**通信作者;E-mail: mhfeng@niglas.ac.cn.

