骨髓间充质干细胞预防手术后肿瘤肺转移动物模型研究
汪 珺,苏晓三,杨 柳,乔 飞,王翼寅,陈 睿
1.昆明医科大学第一附属医院麻醉科,云南 昆明 650031;2.昆明医科大学附属甘美医院生物医学实验中心,云南 昆明 650011
骨髓间充质干细胞预防手术后肿瘤肺转移动物模型研究
汪 珺1,苏晓三2,杨 柳2,乔 飞1,王翼寅2,陈 睿2
1.昆明医科大学第一附属医院麻醉科,云南 昆明 650031;2.昆明医科大学附属甘美医院生物医学实验中心,云南 昆明 650011
背景与目的:近年来研究发现,肿瘤患者术后髓系抑制细胞(myeloid-derived suppressor cells,MDSCs)较术前升高,且其促肿瘤血管生成和肿瘤生长作用增强,骨髓间充质干细胞(bone marrow mesenchymal stem cells,BMSCs)可抑制MDSCs活化与增殖。但围手术期MDSCs的变化与肿瘤术后转移的关系及BMSCs能否通过抑制MDSCs预防手术后肿瘤转移尚不清楚。该研究拟探讨围手术期MDSCs变化与手术后肿瘤转移的相关性及BMSCs对围手术期MDSCs和手术后肿瘤转移的影响。方法:C57BL/6小鼠经尾静脉注射LLC细胞后分为4组:对照组(C组)、麻醉组(A组)、麻醉后开腹组(AL组)及麻醉后开腹并肝叶切除组(ALH组)。AL组小鼠手术后分为2组:术后无治疗组(AL1组)和术后给予同系BMSCs治疗组(ALB组)。流式细胞术检测小鼠外周血单个核细胞(peripheral blood mononuclear cells,PBMCs)中Gr-1+CD11b+细胞的含量;第14天计数小鼠肺表面转移灶。小鼠骨髓细胞体外诱导MDSCs体系中加入BMSCs共培养的方法探讨BMSCs对MDSCs生成的影响。结果:与C、A组相比,AL和ALH组小鼠肺转移灶显著增多(P<0.01);且ALH组较AL组显著增多(P<0.05)。手术后AL和ALH组小鼠PBMCs中Gr-1+CD11b+细胞与C、A组相比显著升高;与AL组比较,ALH组Gr-1+CD11b+细胞显著升高。第14天,AL及ALB组小鼠肺表面转移灶数量分别为38.00±9.57和6.54±1.49,差异有统计学意义(P<0.01)。ALB组小鼠PBMCs中Gr-1+CD11b+细胞与AL1组相比明显降低。小鼠骨髓细胞体外诱导MDSCs体系中加入BMSCs可显著抑制MDSCs的活化和增殖。结论:手术应激诱导MDSCs并促肿瘤肺转移形成,BMSCs可以抑制MDSCs的生成进而抑制手术后肺转移形成。
间质干细胞;髓系细胞;手术后期间;肿瘤转移
手术是治疗肿瘤的首选方法,能改善患者的预后,延长生存期。但围手术期应激反应可抑制患者免疫功能,导致术后肿瘤转移的风险增加[1]。髓系抑制细胞(myeloid-derived suppressor cells,MDSCs)是一群异质细胞,由不同分化阶段的髓系细胞如单核细胞、粒细胞及未成熟的树突状细胞(dendrtic cell,DC)等组成[2]。最早发现MDSCs是在小鼠体内,表达粒细胞和单核细胞的标记Gr-1和CD11b。MDSCs具有很强的免疫抑制作用,是引起肿瘤免疫逃逸的重要细胞群体。另外有研究发现,同系来源的骨髓间充质干细胞(bone marrow mesenchymal stem cells,BMSCs)可抑制MDSCs活化与增殖[3-4]。本研究拟采用小鼠手术诱导肿瘤肺转移模型观察围手术期MDSCs变化与手术后肿瘤肺转移的相关性及围手术期采用同系来源BMSCs对MDSCs和手术后肺转移的影响,从而为预防手术后肿瘤转移提供一定的依据。
1 材料和方法
1.1 细胞系和主要试剂
C57BL/6小鼠Lewis肺癌(lewis lung carcinoma,LLC)细胞系由云南省肿瘤研究所惠赠。小鼠成纤维细胞系NIH-3T3购自中国科学院昆明动物研究所。荧光标记抗体异硫氰酸荧光素(fluorescein isothiocyanate,FITC)-Ly-6G/Ly-6C(Gr-1)(克隆RB6-8C5)和藻红蛋白(phycoerythrin,PE)-CD11b(克隆M1/70)购自美国Becton Dickinson公司。
1.2 实验动物
无特定病原体级C57BL/6小鼠,雌性,8~10周龄,体质量为18~22 g,生产许可证号SCXK(京)2009-0004,购自北京华阜康生物科技股份有限公司。
1.3 实验方法
1.3.1 小鼠BMSCs体外培养
按照参考文献[5]中的方法分离、培养C57BL/6小鼠BMSCs。培养至第3代的BMSCs用于小鼠LLC肺转移治疗。
1.3.2 小鼠肺转移瘤模型的构建和BMSCs治疗
C57BL/6小鼠经尾静脉注射LLC细胞(1.5×104个/小鼠)。2 h后将接种LLC的小鼠随机分为4组:对照组(C组)、麻醉组(A组)、麻醉后开腹组(AL组)及麻醉后开腹并肝叶切除组(ALH组)。A组小鼠按照0.8 mg/kg腹腔注射戊巴比妥钠(购自美国Sigma公司)进行麻醉,AL组小鼠麻醉后无菌条件下沿腹中线切开约3 cm长切口,随后将切口缝合;ALH组小鼠麻醉后无菌条件下沿腹中线切开约3 cm长切口,随后切除左侧一叶肝叶最后将腹部切口缝合。选择8只AL组小鼠待手术后2 h经尾静脉输注BMSCs 2×105个/小鼠,随后每天1次、连续注射3次(ALB组)。未注射BMSCs组则命名为AL1组。于接种LLC后第14天处死小鼠取肺、计数小鼠肺表面肿瘤结节。
1.3.3 流式细胞术检测Gr-1+CD11b+细胞的含量
C、A、AL、ALH及ALB组自LLC接种后第1天开始采集小鼠尾静脉血,采用流式细胞术检测小鼠外周血单个核细胞(peripheral blood mononuclear cells,PBMCs)中Gr-1+CD11b+细胞含量。
1.3.4 体外诱导MDSCs
小鼠MDSCs体外诱导采用参考文献[6]中的方法:C57BL/6小鼠骨髓细胞用含小鼠粒细胞-巨噬细胞集落刺激因子RPMI-1640按5×105个/孔接种至24孔板中,实验组每孔加入C57BL/6来源BMSCs 1×105个/孔(+BMSCs组),设加入RPMI-1640或NIH3T3 1×105个/孔为对照组(分别为media组和+NIH3T3组)。培养24、48和72 h后分别收集悬浮细胞,流式细胞术检测Gr-1+CD11b+细胞含量。
1.4 统计学处理
2 结 果
2.1 手术应激促肿瘤转移
C57BL/6小鼠接种LLC细胞至第14天,C、A、AL和ALH组小鼠肺表面肿瘤转移灶数量分别为(14.00±5.04)、(16.00±4.12)、(38.00±9.57)和(63.00±14.48)。与C、A组相比,AL和ALH组小鼠肺表面肿瘤转移灶显著增多(P<0.01),且ALH组较AL组显著增多(P<0.05,图1)。
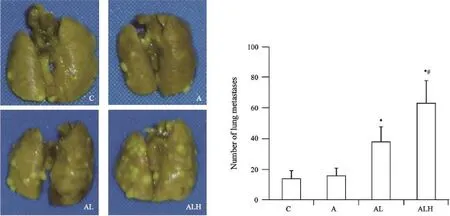
图1 LLC细胞静脉接种第14天,小鼠肺表面肿瘤转移灶数量Fig. 1 The number of tumor metastase on the lung surface 14 days after LLC cells inoculation intravenously
2.2 手术应激促MDSCs活化、增殖
接种LLC细胞的C57BL/6小鼠在手术后通过流式细胞术检测PBMCs中Gr-1+CD11b+细胞。结果显示,与C、A组相比,AL和ALH组于术后第1天即显著升高(P<0.01),且ALH组与AL组相比亦显著升高(P<0.05,图2)。术后第2、3、5、 8、11和14天,AL和ALH组小鼠PBMCs中Gr-1+CD11b+细胞与C、A组相比显著升高;与AL组比较,ALH组Gr-1+CD11b+细胞显著升高。提示荷瘤小鼠外周血中Gr-1+CD11b+细胞随手术应激增加而升高。
2.3 BMSCs通过抑制MDSCs预防手术后肿瘤转移
C57BL/6小鼠接种LLC第14天,AL1及ALB组小鼠肺表面肿瘤转移灶数量分别为(38.00±9.57)和(6.54±1.49),差异有统计学意义(P<0.01,图3A)。接种LLC细胞的C57BL/6小鼠手术后第3天,ALB组小鼠PBMCs中Gr-1+CD11b+细胞与AL组相比显著降低(P<0.01,图3B)。术后第5、8、11和14天,ALB组小鼠PBMCs中Gr-1+CD11b+细胞与AL组相比均显著降低(P<0.05,图3C、D)。
2.4 BMSCs体外抑制MDSCs
对体外培养C57BL/6小鼠骨髓细胞采用流式细胞术检测其中Gr-1+CD11b+细胞的含量。结果显示,体外诱导MDSCs至48和72 h时,BMSCs共培养组Gr-1+CD11b+细胞较Media组及NIH-3T3共培养组显著降低(P<0.05,图4)。
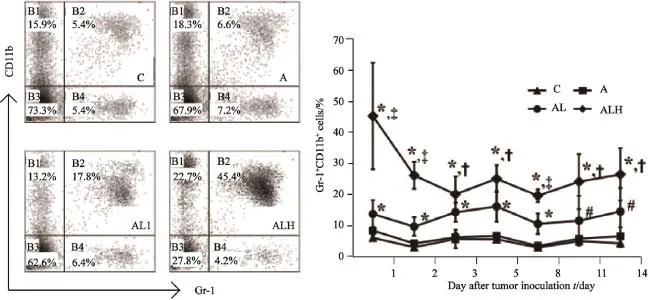
图2 小鼠经尾静脉接种LLC、手术后PBMCs中Gr-1+CD11b+细胞的变化Fig. 2 The changes of Gr-1+CD11b+cells in PBMCs after LLC cells inoculation intravenously and surgical operation
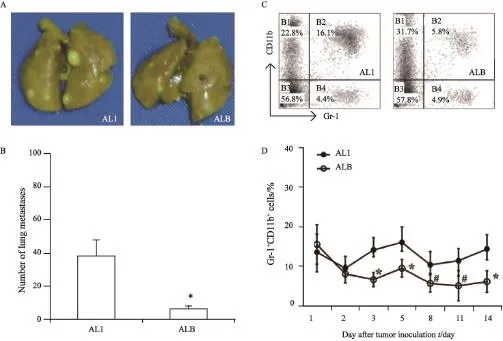
图3 小鼠经BMSCs治疗后第14天,肺表面转移灶数量及PBMCs中Gr-1+CD11b+细胞变化Fig. 3 The numbers of metastases on the lung surface on the 14thday and the changes of Gr-1+CD11b+cells in PBMCs after syngeneic BMSCs treatment
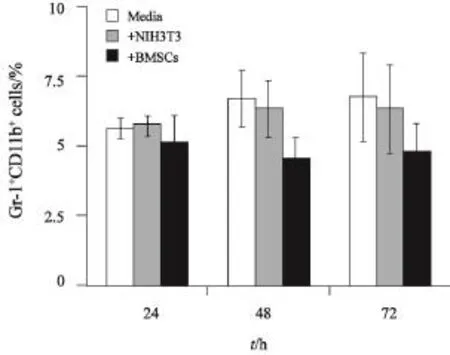
图4 BMSCs对体外诱导MDSCs的影响Fig. 4 The inf l uence of BMSCs on the induction of MDSCs in vitro
3 讨 论
手术后肿瘤是否转移主要取决于肿瘤的转移能力和机体抗转移能力的平衡,虽然肿瘤转移灶在被发现之前通常要经过数月时间的发展,但围手术期这一短暂的时期会对残留或循环中的肿瘤细胞产生巨大的影响[1]。目前已知的是手术后循环中的细胞免疫(cell mediated immunity,CMI)成分如T细胞、自然杀伤(nature killer,NK)细胞迅速下降,术后第3天,CMI抑制达峰值,之后CMI逐渐恢复,至手术后第21天恢复至术前水平[7]。有研究则发现,在术后CMI抑制的同时MDSCs急剧升高,在术后第3天达到峰值,之后逐渐下降[8]。因此,术后CMI的缺失和迅速升高的MDSCs共同为残留或循环中的肿瘤细胞提供了一个极为短暂的时机。MDSCs可能协助循环中的肿瘤细胞侵入靶器官并形成转移灶,而当转移灶生长到超过一定的体积时,此时的肿瘤微环境足以对抗CMI的攻击,即使CMI完全恢复也不可能清除转移灶[9]。
BMSCs最重要的生物学特性就是其强大的免疫调节功能,目前已证实MSCs对T细胞、B细胞、DC细胞、NK细胞、粒细胞和巨噬细胞等均有不同程度的免疫调节能力[10]。BMSCs与 MDSCs均来源于骨髓,在肿瘤或急慢性炎症等疾病发生、发展过程中,BMSCs与MDSCs具有重要的免疫调控功能[11]。在机体处于手术等急性应激状态时,骨髓中BMSCs与MDSCs可以迅速动员并释放至外周组织(如外周血)继而发挥组织修复和免疫调控功能。在此过程中,BMSCs与MDSCs是否存在相互作用目前尚无报道。
本研究通过静脉接种肺癌细胞LLC,随后进行麻醉、开腹及肝叶切除等模拟手术应激促进循环中的肿瘤细胞形成肺转移,观察并验证手术应激与手术后肿瘤转移的相关性。结果发现,随着手术创伤的增加,小鼠肺转移灶的数量有所增加。进而我们通过对手术应激状态下荷瘤小鼠MDSCs的变化动态观察发现手术创伤的加重可增加外周血MDSCs的活化和增殖,即手术应激促进了MDSCs的活化和增殖。肿瘤的转移是一个多步骤的过程,而MDSCs在这个过程的多个环节中均发挥着重要的作用,在肿瘤发生的早期,MDSCs可以直接诱导上皮-间质转化的发生,在侵袭前沿降解细胞基质,继而参与到转移前微环境的形成,形成肿瘤细胞定植的微环境,在肿瘤细胞到达转移部位后,MDSCs又通过分泌一系列细胞因子促进转移灶的最终形成[12]。为证实手术通过诱导MDSCs活化、增殖从而促进手术后肿瘤转移,本研究采用同系小鼠来源的BMSCs治疗并观察对手术后小鼠外周血MDSCs水平及肿瘤肺转移的影响。结果发现,BMSCs治疗后小鼠PBMCs中MDSCs显著降低,而手术后小鼠肺转移灶也明显减少,提示BMSCs可能通过抑制手术应激状态下MDSCs的活化、增殖进而抑制手术后肿瘤的转移。
综上所述,手术应激反应可刺激MDSCs活化、增殖并释放至外周血中,这些MDSCs在机体术后抗感染及促进创伤修复过程中起着关键作用,但如果手术未能完全清除肿瘤或手术时肿瘤已发生微转移,则MDSCs可能通过迁移至肿瘤局部并最终导致肿瘤复发和(或)转移。但手术后MDSCs促肿瘤转移的具体机制尚待进一步研究。
[参 考 文 献]
[1] HOROWITZ M, NEEMAN E, SHARON E, et al. Exploiting the critical perioperative period to improve long-term cancer outcomes[J]. Nat Rev Clin Oncol, 2015, 12(4): 213-226.
[2] PARKER K H, BEURY D W, OSTRAND-ROSENBERG S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment[J]. Adv Cancer Res, 2015, 128: 95-139.
[3] SU X S, ZHANG L, YE J S, et al. Bone marrow mesenchymal stem cells suppress ascitogenous hepatoma progression in BALB/c mouse through reducing myeloid-derived suppressor cells[J]. Biomed Mater Eng, 2015, 25(1 Suppl): 167-177.
[4] ZHAGN L, SU X S, YE J S, et al. Bone marrow mesenchymal stem cells suppress metastatic tumor development in mouse by modulating immune system[J]. Stem Cell Res Ther, 2015, 6: 45.
[5] OTSU K, DAS S, HOUSER S D, et al. Concentrationdependent inhibition of angiogenesis by mesenchymal stem cells[J]. Blood, 2009, 113(18): 4197-4205.
[6] YOUN J I, NAGARAJ S, COLLAZO M, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice[J]. J Immunol, 2008, 181(8): 5791-5802.
[7] JIANG L, NICK A M, SOOD A K. Fundamental principles of cancer biology: Does it have relevance to the perioperative period?[J]. Curr Anesthesiol Rep, 2015, 5(3): 250-256.
[8] WANG J, SU X S, YANG L, et al. The influence of myeloid derived suppressor cells on angiogenesis and tumor growth after cancer surgery[J]. Int J Cancer, 2016, 138(11): 2688-2699.
[9] BEAVIS P A, SLANEY C Y, KERSHAW M H, et al. Enhancing the efficacy of adoptive cellular therapy by targeting tumor-induced immunosuppression[J]. Immunotherapy, 2015, 7(5): 499-512.
[10] ENGLISH K. Mechanisms of mesenchymal stromal cell immunomodulation[J]. Immunol Cell Biol, 2013, 91(1):19-26.
[11] BIANCHI G, BORGONOVO G, PISTOIA V, et al. Immunosuppressive cells and tumour microenvironment: focus on mesenchymal stem cells and myeloid derived suppressor cells[J]. Histol Histopathol, 2011, 26(7): 941-951.
[12] CONDAMINE T, RAMACHANDRAN I, YOUN J I, et al. Regulation of tumor metastasis by myeloid-derived suppressor cells[J]. Annu Rev Med, 2015, 66: 97-110.
Bone marrow mesenchymal stem cells prevent pulmonary tumor metastasis after surgery in a mouse model
WANG Jun1, SU Xiaosan2, YANG Liu2, QIAO Fei1, WANG Yiyin2, CHEN Rui2(1. Department of
Anesthesiology, the First Affiliated Hospital of Kunming Medical University, Kunming 650031, Yunnan Province, China; 2. Biomedical Research Center, the Affiliated Ganmei Hospital of Kunming Medical University, Kunming 650011, Yunnan Province, China)
Background and purpose: In recent years, the studies indicated that postoperatively induced myeloid-derived suppressor cells (MDSCs) were qualified with potent proangiogenic and tumor-promotive ability. Bone marrow mesenchymal stem cells (BMSCs) significantly inhibited the induction and proliferation of MDSCs. However, the relationship of MDSCs and tumor metastasis during perioperative period, and whether BMSCs could prevent tumor metastasis through inhibiting MDSCs are not clarif i ed. This study aimed to investigate the change of MDSCs during perioperative period and its correlation with tumor metastasis after surgery, and the inf l uence of BMSCs on the induction of MDSCs and the development of postoperative tumor metastasis. Methods: LLC cells were injected intravenously into C57BL/6 mice. Two hours later, these mice were divided into 4 groups: controls (C group); micegiven anesthesia (A group); mice given anesthesia and laparotomy (AL group) and mice given anesthesia, laparotomy, and hepatic lobectomy (ALH group). The AL mice were divided into 2 groups after surgical operation: the AL mice without treatment (ALL group) and the AL mice treated with syngeneic BMSCs (ALB group). The percentage of Gr-1+CD11b+cells in peripheral blood mononuclear cells (PBMCs) was detected by flow cytometry. The numbers of metastases on the lung surface were counted on the 14thday after LLC infusion. BMSCs were also co-cultured in vitro with myeloid cells in order to illustrate the ef f ects of BMSCs on the generation of MDSCs. Results: The numbers of lung metastases in AL and ALH group signif i cantly increased as compared with C and A group (P<0.01). The number of lung metastases in ALH group signif i cantly increased as compared with AL group (P<0.05). The percentage of Gr-1+CD11b+cells in PBMCs during postoperative period signif i cantly increased in AL and ALH group as compared with C and A group, and the percentage of Gr-1+CD11b+cells in ALH group also signif i cantly increased as compared with AL group. The numbers of lung metastases in AL and ALB group were (38.00±9.57) and (6.54±1.49), the dif f erence was statistically signif i cant (P<0.01) on day 14 after LLC infusion. Meanwhile, the percentage of Gr-1+CD11b+cells in ALB group signif i cantly decreased as compared with AL1 group. This study also demonstrated that BMSCs inhibited the induction and proliferation of MDSCs from myeloid cells in vitro. Conclusion: Surgery stress induces MDSCs and promotes tumor metastasis. Syngeneic BMSCs could inhibit the generation of MDSCs and further suppress tumor metastasis after surgery.
Mesenchymal stem cell; Myeloid cells; Postoperative period; Neoplasm metastasis
SU Xiaosan E-mail: suxs163@163.com
10.19401/j.cnki.1007-3639.2017.02.002
R73-37
A
1007-3639(2017)02-0089-06
2016-09-02
2016-12-20)
国家自然科学基金资助项目(31360223)。
苏晓三 E-mail:suxs163@163.com

