Effect of periprosthetic fracture on hip function after femoral neck-preserving total hip arthroplasty: study protocol for a prospective, single-center, self-controlled trial with 2-year follow-up
Di Qin, Yong-tai Han, Hui-jie Li
Department of Bone Diseases, Third Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, China
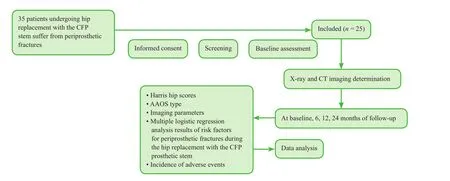
Figure 1: Flow chart of the study protocol.
Introduction
History and current related studies
Periprosthetic fractures are challenging for orthopedic surgeons (Yu et al., 2008; Barut et al., 2015; Frenzel et al.,2015; Kempthorne et al., 2015; Massari et al., 2015), and are more likely to appear during hip replacement with a cementless prosthesis (Petersen et al., 1998). Hip replacement with a collum femoris preserving (CFP) prosthetic stem(Waldemar Link, Hamburg, Germany) allows preservation of as much of the femoral neck and bone tissue as possible for future prosthetic revision (Pipino, 2004). However,although hip replacement with the CFP prosthetic stem can reduce complications and pain, intraoperative periprosthetic fractures are more likely to occur, which negatively impacts functional recovery (Shen et al., 2004; Klein et al., 2005;Huang et al., 2014).
Main objective
We aim to provide information on reducing the incidence of periprosthetic fractures during hip replacement with the CFP prosthetic stem by analyzing the risk factors for periprosthetic fractures and their effects on hip functional recovery.
Novelty of this study
There are currently no imaging staging criteria for periprosthetic fractures occurring during hip replacement with the CFP prosthetic stem. Hence, we will develop staging criteria for periprosthetic fractures to provide an objective standardized reference for timing and surgical approach in hip replacement, aiming to hasten hip functional recovery.
MethodS/deSign
Study design
A prospective, single-center, self-controlled, open-label clinical trial.
Study setting
The Third Hospital of Hebei Medical University, Hebei Province, China
Study procedures
We will analyze data from 25 patients with periprosthetic fractures after hip replacement with the CFP prosthetic stem at the Department of Orthopedic Surgery, the Third Hospital of Hebei Medical University, who will be followed up for 24 months. Parametric analysis of the proximal medullary cavity of the femur on radiography and CT will be performed preoperatively and 6, 12 and 24 months postoperatively. Figure 1 shows a flow chart of the study protocol.
Inclusion criteria
Patients of either sex meeting all of the following criteria will be included in the study:
· Diagnostic and staging criteria in line with the Expert Consensus on Diagnosis and Treatment of Adult Femoral Head Necrosis (2012 edition) (Department of Orthopedics, Chinese Medical Association, 2012)
· Femoral head necrosis assessed as Association Research Circulation Osseous (ARCO) stage III-IV (Gardeniers,1993)
· Unilateral femoral head necrosis
· Age < 50 years
Exclusion criteria
Patients with any of the following criteria will be excluded from the study:
· Advanced osteoarthritis
· Secondary osteoarthritis due to acetabular dysplasia
· Ankylosing spondylitis involving the hip joint
· Rheumatoid arthritis
· In flammatory in flammation of the hip joint
· Tumor lesions in the hip joint
· Unable or unwilling to sign the informed consent
Baseline data
Baseline data, including demographic data and general disease history, will be collected from the participants(Table 1).

Table 1: Baseline analysis
Sample size
Based on our previous experience, we assumed that the Harris hip scores would be increased to over 70 points at 6 months postoperatively. Consideringα= 0.05 (two-sided),β= 0.1, and power = 90%, the necessary sample size was calculated using PASS 11.0 software (NCSS, Kaysville, UT,USA) to ben= 21. With a predicted dropout rate of 20%,the required sample size was calculated asn= 25. After application of the inclusion and exclusion criteria, a final total of 25 patients will be included in this study.
Recruitment
The first author will search the case database of the Third Hospital of Hebei Medical University to identify all eligible patients with periprosthetic fractures who meet the inclusion and exclusion criteria. Either the patients or their relatives will be contactedviatelephone. These eligible patients have developed periprosthetic fractures when they undergo hip replacement with the CFP prosthetic stem. All participants will be informed of the study protocol and provide written consent. We then will analyze their medical records and 24-month follow-up results.
Blinding
This is an open-label trial. All participants and investigators will be aware of the grouping information.
Interventions
(1) Joint prosthesis: the CFP prosthetic stem enables preservation of the femoral head and neck. Considering the biomechanical characteristics of the hip, the CFP stem is designed to strengthen the surrounding bone tissue of the femoral neck, achieving a sustained and stable fixation.Owing to its good anatomical design, the prosthesis allows physiological stress conduction, which creates biomechanical integration of the prosthesis and the proximal femur. Use of the CFP stem can maintain the blood supply around the femoral neck and retain a large amount of bone tissue in the metaphysis, which enables long-term bone ingrowth to achieve biomechanical fixation of the prosthesis. Minimizing the loss of bone tissue provides a structural basis for revision surgery.
The CFP stem comprises a prosthetic stem made from titanium alloy with titanium coating (porous surface with hydroxyapatite coating), a prosthetic neck made of cobaltchromium-molybdenum alloy, and a prosthetic femoral head made of alumina ceramics.
(2) Hip replacement with the CFP stem (femoral neck preservation): the patients will have an indwelling catheter placed preoperatively and will be generally anesthetized via tracheal cannula. Hip replacement will be performed with the patient in the contralateral supine position, to keep the torso perpendicular to the operating table. The sacrum and pubic symphysis will be fixed with a waist block, allowing the hip joint to move freely. A cushion will be placed below the anterior superior iliac spine and the axilla, and the perineum will be covered sterilely with a protective membrane. The affected limb will be suspended following abduction and external rotation, and then routinely surgically prepared. A posterolateral arc-shaped incision of 10-15 cm will be made at the point 5 cm below the posterior superior iliac spine, starting from the gluteus maximus through the greater trochanter, turning to the femoral stem (extending about 5 cm downwards). Following successive exposure of skin, subcutaneous tissues and femoral fascia, the gluteus maximus will be bluntly isolated along the muscle fibers from the sciatic nerve. The bilateral gluteus maximus will be then separated through flexion, abduction and internal rotation of the affected lower limb, to expose the hip external rotators and adipose tissue on the muscle surface attached to the intertrochanteric fossa. The hip external rotator of the intertrochanteric fossa will be taken as a dead point to cut off the piriformis, gemellus superior, obturator muscle,and gemellus inferior in order. The femoral head will be then dislocated through a longitudinal incision of the joint capsule along the femoral neck. The femoral head will be partially resected in line with the prosthetic requirements.The acetabulum will be drawn at 0°, 120° and 240° angles to be fully exposed by straightening the affected lower limb.The joint capsule and osteophytes around the acetabulum will be removed, to maintain the appropriate range of limb abduction and anteversion. Acetabular reaming will be done using different sized reamers in proper order (from small to large) until the cartilage surface will be bleeding.Subsequently, the acetabular outer and inner cups will be set up. An intramedullary reamer will be used to expand the medullary cavity along the medullary axis following excessive flexion, internal rotation, and adduction of the affected lower limb. Prosthesis and femoral head models will be installed, the length of bilateral lower limbs and movement of the hip joint will be determined by reduction measures, and the hip joint will be checked for dislocation.Selection of the prosthesis and femoral head will be determined depending on the results of trial assembly, and the fixation method will be consequently selected according to the type of prosthesis (biotype or cement-based). Finally, a drainage tube will be placed followed by layered suturing.(3) Types of fractures and the corresponding measures undertaken in the seven pre-experimental cases:
· In one case of American Academy of Orthopaedic Surgeons (AAOS) type II fracture, the fracture line was located above the small tuberosity, and as the prosthesis seems to be stabilized intraoperatively, no particular treatment was required. Appropriate postoperative rehabilitation exercises were performed.
· In one case of AAOS III fracture, the fracture line stretched over the intertrochanteric connection. Intraoperatively, the stability of the prosthesis was impacted by the fracture; therefore, strong fixation with a bundling belt was performed. Adequate postoperative rehabilitation exercises were performed.
· In four cases of AAOS IV fracture, simple split oblique fractures had occurred, with the fracture line located in the lateral cortex of the proximal femur and a small shift of the prosthesis at the distal end. Therefore, no particular surgical treatment was required. Postoperatively, the patients were asked to carry out non-weight-bearing exercise for 4 weeks, followed by weight-bearing exercises with the assistance of crutches.
· In one case of AAOS V fracture, an oblique comminuted fracture of the proximal femur occurred. The fracture line was located 3 cm distal to the prosthesis, and there was no obvious prosthetic shift. Therefore, an intraoperative locking plate + bundling belt were used for reinforcement. The patient was required to carry out non-weightbearing exercise for 4 weeks, followed by weight-bearing exercises with the assistance of crutches.
(4) Collection of imaging data: radiographic and CT data will be collected from each patient preoperatively and 6,12, and 24 months postoperatively, plus data from one preoperative MRI scan.
· Radiograph acquisition: anteroposterior and frog-leg lateral radiographs of the hip as well as full-length radiographs of the bilateral lower extremities.
· CT three-dimensional image acquisition: preoperative CT scanning of the bilateral hip joints using SOMATOM Sensation 64-row CT (Siemens, Germany), which was used for coronal, sagittal and axial three-dimensional reconstruction.
· MRI three-dimensional image acquisition: preoperative
coronal, sagittal and axial MRI images of the bilateral hips will be acquired using MAGNETOM Verio 3.0 T-MRI (Siemens). The acquisition sequence is: T1WI/TSE: TR = 478 ms; TE = 20 ms; layer thickness = 3 mm;interlayer spacing = 0.9 mm; FOV = 380 × 320 as well as T2WI/TIRM: TR = 5,000; TE = 29; slice thickness =3 mm; interlayer spacing = 0.9 mm; FOV = 380 × 320.
Outcome measures
Primary outcome
Harris hip scores will be recorded preoperatively and 6, 12 ,and 24 months postoperatively. The Harris hip score ranges from 0-100 points, where a score ≥ 90 points indicates excellent function, 80-89 points indicates good function,70-79 points indicates fair function, and < 70 points indicates poor function.
Secondary outcomes
Imaging parameters determined by CT and radiography performed preoperatively and 6, 12, and 24 months postoperatively include parameters of the proximal and mesal medullary cavity of the fractured femur (width of the medullary cavity at the point 20 mm above the midpoint of the small trochanter (T + 20), at the midpoint of the small trochanter(T), at the point 20 mm below the midpoint of the small trochanter (T - 20), and at the fracture line (F), as well as width of the corresponding prostheses), intertrochanteric width (RLTF), femoral neck-stem angle (CA), femoral neck anteversion (FNA), femoral neck length (NL), ratio of the distal end of the prosthesis to the medullary space (R), and height of the femoral calcar (CF).
Three-dimensional anatomical measurement: DICOM image files acquired by imaging equipment will be imported into Digimizer V3.8.1.0 (MedCale Software, USA) for image measurement and analysis.
In ARCO III patients, three-dimensional anatomicalchanges will be analyzed by longitudinal assessment of follow-up data. The effects of different affected parts and different degree of collapse on the hip joint function will be determined by measurement of Harris hip scores.
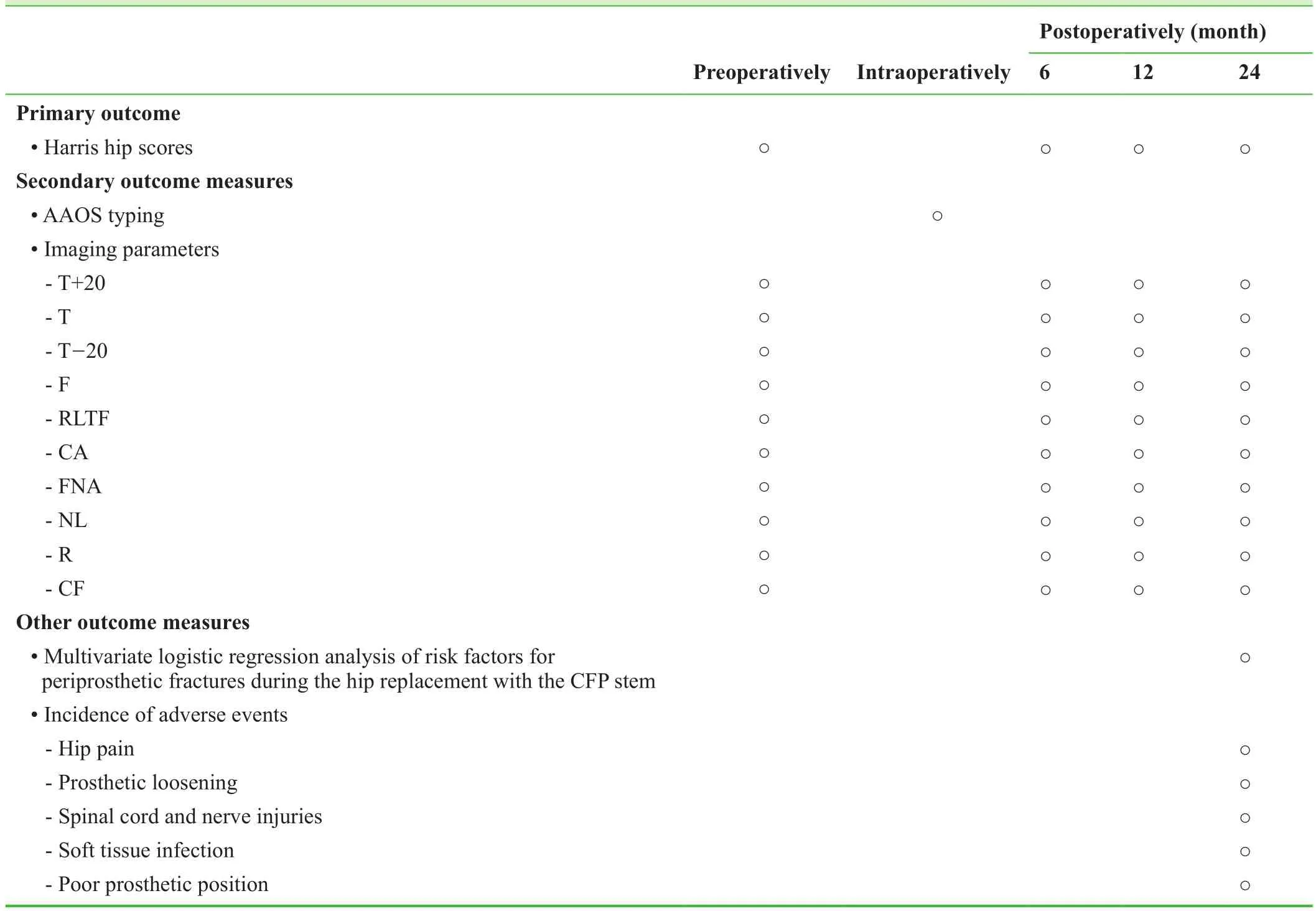
Table 2: Schedule for outcome measures
In ARCO IV patients, three-dimensional anatomical data related to anatomical reconstruction of the hip joint before and after surgery will be measured. Anatomical measurements of the normal and affected hip joints will be compared. Signi ficance and value of anatomical reconstruction,functional reconstruction, and prosthesis selection during hip replacement will be analyzed by determination of Harris hip scores before and after treatment.
Other measures
· Multivariate logistic regression analysis of risk factors for periprosthetic fracture during hip replacement with the CFP prosthesis, which may include improper choice of osteotomy plane, proximal femoral malformations, type of prosthesis, poor implanting angle, and femoral neck sclerosis.
· The incidence of adverse events at 24 months postoperatively will be recorded.
Table 2 shows the schedule for outcome measures.
Adverse events
· Adverse events occurring during the follow-up period will include hip pain, prosthetic loosening, spinal cord and nerve injuries, soft tissue infection, and poor prosthetic position.
· If severe adverse events occur during the follow-up period, details including the date of occurrence, type of adverse events and measures taken will be recorded and reported to the principal investigator and the institutional review board within 24 hours.
Data collection, management, analysis, and open access
Data collection
Clinical data, including demographic data, disease diagnosis, accompanying diseases, allergic history (drug allergy)and adverse events, will be collected and summarized using standardized case report forms. These data will be processed using Epidata software and electronically input using double entry system.
Data management
After database con firmation, only the project manager will be able to access the database. The locked data will be unable to be altered and were preserved by the Third Hospital of Hebei Medical University.
Data analysis
All data will be statistically analyzed by professional statisticians who will be responsible for completing an outcome analysis report that will be submitted to the project manager. An independent data monitoring committee will be responsible for data monitoring and management throughout the entire trial to ensure scienti fic accuracy,stringency, authenticity, and integrity.
Open data
Published data will be released at http://www. figshare.com.
Statistical analysis
All data will be statistically analyzed by statisticians using SPSS 21.0 software (IBM, Armonk, NY, USA) in line with the intention-to-treat principle. Normally distributed measurement data will be expressed as means, standard deviations, minimums, and maximums, while non-normally distributed data will be expressed as lower quartiles, medians,and upper quartiles. The Wilcoxon matched paired test will be used for comparative analysis of Harris hip scores and imaging parameters preoperatively and 6, 12, and 24 months postoperatively. Then, multivariate logistic regression analysis will be used to calculate the regression coef ficient,OR, and 95%CIwith the Harris hip score as the dependent variable for each imaging parameter that has signi ficant difference. The statistical signi ficance level will beα= 0.05.
Auditing
Trial progress will be reported to the ethics committee of the Third Hospital of Hebei Medical University every 3 months and the trial status will be updated in the registration database after each report.
Confidentiality
· Valuable trial data will be transcribed, dated, and uploaded to a dedicated computer by two staff members,and then scheduled, checked, locked by an investigator.
· These data will be not altered after locking. Unauthorized persons will be unable to access the database.
· Data regarding this trial protocol will be preserved by the Third Hospital of Hebei Medical University.
Trial Status
Recruitment is ongoing at the time of submission.
Discussion
Significance of this study
Detailed preoperative planning and prediction of surgical risks are crucial to reduce the incidence of periprosthetic fracture during hip replacement. We will explore and analyze the risk factors for periprosthetic fracture during hip replacement with the CFP stem.
Advantages and limitations of this study
Advantages of the study include the assessment of objective imaging parameters measured on CT and radiography in patients with periprosthetic fractures during hip replacement,combined with the assessment of hip function recovery. This allows us to fully analyze the risk factors for periprosthetic fractures during hip replacement.
Limitations include the small sample size and use of single indicators, which may affect the accuracy of the results.
Novelty of this study
This prospective study aims to analyze the risk factors for periprosthetic fractures during femoral neck preserving hip replacement. These factors are expected to be used to develop preoperative planning and predict surgical risks to reduce the intraoperative incidence of periprosthetic fracture and improve functional recovery of the hip in patients scheduled for hip replacement with the CFP stem.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms. In the form the patients will give their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
None declared.
Author contributions
DQ conceived and designed the study protocol, and wrote the paper. YTH reviewed the manuscript. HJL participated in the trial execution. All authors approved the final version of this paper.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
Barut N, Anract P, Babinet A, Biau D (2015) Peri-prosthetic fractures around tumor endoprostheses: a retrospective analysis of eighteen cases. Int Orthop 39:1851-1856.
Department of Orthopedics, Chinese Medical Association (2012)Expert consensus on diagnosis and treatment of adult femoral head necrosis. Zhonghua Guke Zazhi 32:185-192.
Frenzel S, Vécsei V, Negrin L (2015) Periprosthetic femoral fractures-incidence, classi fication problems and the proposal of a modi fied classi fication scheme. Int Orthop 39:1909-1920.
Gardeniers JWM (1993) The ARCO perspective for reaching one uniform staging system of osteonecrosis. Bone circulation and vascularization in narmal and pathological conditions. New York:Plenum, USA.
Huang X, Zhang Y, Wang Q (2014) Medium- and long-term effectiveness of third-generation ceramic-on-ceramic total hip arthroplasty for end-stage hip disease. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 28:669-672.
Kempthorne JT, Ailabouni R, Raniga S, Hammer D, Hooper G(2015) Occult infection in aseptic joint loosening and the diagnostic role of implant sonication. Biomed Res Int 2015:946215.
Klein GR, Parvizi J, Rapuri V, Wolf CF, Hozack WJ, Sharkey PF,Purtill JJ (2005) Proximal femoral replacement for the treatment of periprosthetic fractures. J Bone Joint Surg Am 87:1777-1781.
Massari L, Osti R, Lorusso V, Setti S, Caruso G (2015) Biophysical stimulation and the periprosthetic bone: is there a rationale in the use of pulsed electromagnetic fields after a hip or knee implant? J Biol Regul Homeost Agents 29:1013-1015.
Petersen MB, Gramkow J, Retpen JA, Rechnagel K, Solgaard S(1998) Non-cemented acetabular cup in hip arthroplasty. Prosthesis survival and clinical results after 1-8 years. Ugeskr Laeger 160:4772-4775.
Pipino F (2004) CFP Prosthesic stem in mini-invasive total hip arthroplasty. J Orthop Trauma 4:165-171.
Shen J, Sun CT, Huang GY (2004) Evaluation of the postoperative quality of life in the elderly over 80 years old who underwent hip hemiarthroplasty for femoral neck fracture. Zhonghua Wai Ke Za Zhi 42:1409-1411.
Yu L, Zhang CH, Guo T, Ding H, Zhao JN (2016) Middle and longterm results of total hip arthroplasties for secondary post-traumatic arthritis and femoral head necrosis after acetabular fractures.Zhongguo Gu Shang 29:109-113.
 Clinical Trials in Orthopedic Disorder2017年1期
Clinical Trials in Orthopedic Disorder2017年1期
- Clinical Trials in Orthopedic Disorder的其它文章
- Information for Authors -Clinical Trials in Orthopedic Disorders
- Genetic risk factors of degenerative intervertebral disc disease:a case-control study
- MRI appearance of injured ligaments and/or tendons of the ankle in different positions: study protocol for a single-center,diagnostic clinical trial
- New bone fixation plate for the repair of avulsion fracture of the tibial attachment of the posterior cruciate ligament: study protocol for a prospective, open-label, self-controlled, clinical trial
- Risk factors for ligamentum flavum hypertrophy in lumbar spinal stenosis patients from the Xinjiang Uygur Autonomous Region, China: protocol for a retrospective, single-center study
- Effect of tourniquet use during total knee arthroplasty on global inflammatory cytokine changes associated with ischemiareperfusion injury
