先天免疫受体介导的神经炎症在神经系统疾病中的作用
董 渊*,李晓恒*,侯 琳,程金波
(1.青岛大学医学部,山东青岛 266071;2.首都医科大学脑重大疾病研究院,北京 100069;3.军事医学研究院军事认知与脑科学研究所,北京 100850)
神经炎症是指发生在中枢神经系统(central nervous system,CNS)及周围神经系统中的炎症反应。感染、外伤、有毒代谢物和自身免疫异常等多种因素均可引起神经炎症的发生[1]。大量的研究发现,多种神经退行性疾病的发生与神经炎症密切相关[2],如阿尔茨海默病(Alzheimer disease,AD)[3-4]和帕金森病(Parkinson disease,PD)[5]。在AD中,神经炎症不仅是由β淀粉样蛋白(β-amyloid protein,Aβ)斑块和神经原纤维缠结引起的组织反应,同时作为一种重要的致病因素,在疾病的发生发展过程中起到重要作用[3]。在脑损伤疾病,如脑卒中和创伤性脑损伤中,神经炎症参与损伤修复和免疫防御的同时,其诱导产生的细胞增生与脑损伤的不良预后显著相关[6]。此外,神经炎症也参与抑郁症等精神疾病的发生[7]。
神经炎症主要由神经系统中促炎细胞因子介导,包括肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)、白细胞介素 1β(interleukin-1β,IL-1β)、IL-18、IL-6和Ⅰ型干扰素(interferon,IFN)等。细胞因子是一类调控细胞炎症、信号转导和细胞生长的蛋白,其中趋化因子可调控细胞迁移,并在感染和损伤中起到招募免疫细胞的作用[8]。适当的神经炎症水平是神经系统先天免疫防线中重要的一部分。然而,当神经炎症反应过度且持续地发生时,大量释放的促炎细胞因子,导致神经元的变性和退化、血管内皮细胞的损伤和血脑屏障的破坏,使得外周免疫细胞浸润,造成神经损伤[8]。因此,神经炎症犹如双刃剑,在多种神经退行性疾病、精神疾病和脑损伤中扮演重要角色。先天免疫受体在神经炎症的发生过程中发挥着至关重要的作用。本综述重点总结并讨论参与CNS炎症的主要免疫受体在神经疾病中的作用,以及目前靶向神经炎症的药物研发研究进展。
1 Toll样受体
Toll样受体(Toll-like receptors,TLR)是先天免疫系统中重要的一类模式识别受体(图1)。TLR的激活可经由联接蛋白髓样分化初次应答因子88(myeloid differentiation primary response 88,MYD88)激活下游NF-κB信号通路,调控促炎细胞因子,如TNF-α,IL-1β和IL-6的表达;或经由β干扰素Toll/白细胞介素1受体同源结构或衔接蛋白(TIR-domain-containing adapter-inducing interferon-β,TRIF)通路激活干扰素调节因子3,引起Ⅰ型IFN如IFN-α和IFN-β的表达。目前共发现13种TLR,其中有10种(TLR1~TLR10)在人类细胞中有表达,它们主要以二聚体的形式发挥免疫识别受体的作用。TLR家族受体表达于细胞的细胞膜和胞内体膜上,识别来自微生物序列保守的病原相关分子模式和来自细胞内由炎症和损伤引起的损伤相关分子模式。在CNS中,TLR主要由神经胶质细胞表达,其中小胶质细胞和星形胶质细胞表达TLR1~TLR9,在少突胶质细胞中有TLR2和TLR3的表达[9],TLR在神经元中亦有表达,提示TLR在神经系统中的免疫应答及胶质细胞和神经元的相互作用中起到重要作用[10]。大量的研究发现,多种神经系统疾病与TLR参与的神经炎症密切相关。本文将从神经退行性疾病、精神疾病及脑损伤等几个方面,总结TLR参与神经系统疾病的研究进展。
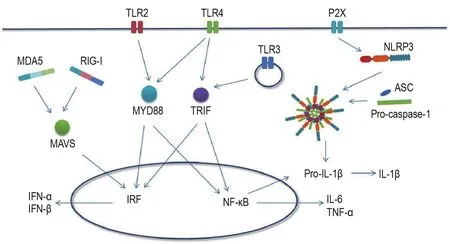
图1 先天免疫识别受体调控细胞因子的释放.TLR:Toll样受体;MYD88:联接蛋白髓样分化初次应答因子88;TNF-α:肿瘤坏死因子α;IL-1β:白细胞介素1β;TRIF:β干扰素Toll/白细胞介素1受体同源结构域衔接蛋白;IRF:干扰素调节因子;IFN:干扰素;NLRP3:NOD样受体蛋白3;ASC:凋亡相关点样蛋白;caspase:胱天蛋白酶;RIG-I:视黄酸诱导基因蛋白I;MDA5:黑素瘤分化相关蛋白5;MAVS:线粒体抗病毒蛋白.
1.1 神经退行性疾病
研究表明,TLR家族受体蛋白在Aβ斑块周围表达显著升高,包括 TLR2,TLR4,TLR5,TLR7 和TLR9等多种类型,提示其在AD发病过程中起到重要作用[11-14]。Aβ注射至小鼠海马体中可引起小胶质细胞中TLR2的表达增加,TLR2的缺失可抑制Aβ引起的小胶质细胞激活和由此引发的神经炎症[15]。然而,在另一项研究中,TLR2的缺失却增加了β淀粉样前体蛋白/早老素1(amyloid-β precursor protein/presenilin-1,APP/PS1)转基因小鼠脑内Aβ的含量,并加剧了小鼠认知能力的损伤[16]。TLR4功能缺失性突变却可抑制Aβ引起小胶质细胞激活和促炎细胞因子的释放,从而抑制了神经炎症和神经损伤的发生,因此在AD的发病中起到保护作用[17]。TLR4可介导小胶质细胞与Aβ的结合,并引起细胞吞噬[18],同时Aβ寡聚体和纤维又可通过CD36引起TLR4和TLR6的聚合,激活下游神经炎症[19]。此外,神经元中TLR2和TLR4参与了Aβ和脂质过氧化物引起的神经细胞凋亡[20]。以上这些研究揭示,TLR在神经炎症和神经退行性疾病中起到重要作用。一方面,TLR介导小胶质细胞吞噬Aβ,加快蛋白沉积的清除,保护神经元;另一方面,TLR引起的小胶质细胞激活和神经炎症促进了神经损伤的发生,反而促进AD病情的发展。
在体过表达或体外使用α突触核蛋白刺激可以导致小胶质细胞活化,同时伴随TLR2表达升高和神经炎症的增加[21],表明TLR2在PD发病过程中具有重要作用。同样,TLR4的表达在PD患者脑中显著增加[22],机制上TLR4可促进小胶质细胞对α突触核蛋白的吞噬,因此保护黑质区多巴胺神经元,但同时TLR4又可通过介导神经炎症加剧1-甲基-4-苯基-1,2,3,6-四氢吡啶(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine,MPTP)诱导的PD模型小鼠的神经损伤[23-24]。
上述研究提示,TLR从不同方面影响了神经退行性疾病的发生发展:一方面,Aβ和α突触核蛋白等蛋白的沉积,通过TLR激活小胶质细胞并引起神经炎症进而造成神经损伤;另一方面,TLR也在调控小胶质细胞清除蛋白沉积中起到重要作用,因此具有神经保护的功能。
1.2 精神疾病
在抑郁症的研究中发现,慢性温和应激后,TLR4激活导致的神经炎症在抑郁症的发病中起到重要作用[25]。临床研究发现,TLR4的激活还与抑郁症和焦虑症引起的肠易激综合征相关[26]。在躁郁症(bipolar disorder,BD)的临床研究中发现,TLR1,TLR2,TLR4和TLR6以及这些受体介导的炎症反应的增强与BD的发生密切相关[27-28],其中,TLR2作用机制可能与调控糖原合成酶激酶3β有关[29]。
1.3 脑损伤
在缺血性脑卒中,TLR也发挥重要作用。脑卒中患者中TLR2和TLR4的表达量、细胞因子IL-1β,IL-6和TNF-α的水平与预后不良程度显著相关[30]。脑缺血再灌注(cerebral ischemia/reperfusion,I/R)模型小鼠皮质和海马区的TLR2和TLR4的含量显著上升,TLR2或TLR4的缺失或抑制可显著降低I/R模型中脑损伤程度[31-33],这部分功能可能来自于神经元表达的TLR2[31,34]。研究发现,TLR4的激活不会增加神经细胞的死亡,低水平的激活反而能促进神经元的生存[35],然而当其过度激活,由此介导的大量神经炎症可能是脑卒中神经损伤的重要来源[36]。TLR8在脑卒中患者的血液中表达量的上升与患者预后的不良程度显著相关,伴随更大的脑梗死面积和严重的神经炎症反应[37]。同样在模型小鼠中,TLR8表达发生显著上调,并加重脑卒中引起的神经损伤[38]。
总之,TLR作为重要的先天免疫受体,参与多种神经系统疾病(包括神经退行性疾病、精神疾病和脑卒中引起的脑损伤)的发生。TLR的激活,一方面能调控神经胶质细胞清除蛋白沉积和损伤的神经元,并因此具有神经保护作用;另一方面,其介导的神经炎症反应在多种神经系统疾病的发展过程中起到了放大作用,并加重神经损伤。
2 Nod样受体
Nod样受体(Nod-like receptors,NLR)是一类胞浆内的免疫识别受体,在先天免疫系统中起到重要作用(图1),NLR激活介导的神经炎症与多种神经疾病的发生密切相关。本部分将主要总结并探讨NLR参与神经退行性疾病和精神疾病的研究进展。
2.1 神经退行性疾病
AD患者脑内神经元NLRP1(NACHT,LRR and PYD domains-containing protein 1)的表达发生显著上调,同时伴有胱天蛋白酶6表达升高[39]。NLRP1炎症小体可激活胱天蛋白酶1和6,并引起神经轴突的变性[40]。AD患者中,NLRP1和NLRP3炎症小体激活,其下游调控的细胞因子IL-1β和IL-18水平显著增加[41]。在巨噬细胞中,Aβ可导致溶酶体损伤,诱导NLRP3炎症小体的激活和IL-1β的激活和释放。在CNS中,NLRP3由小胶质细胞表达,Aβ可激活小胶质细胞,促进IL-1β的释放[42],说明NLRP3炎症小体作为Aβ感受器,在Aβ吞噬及溶酶体损伤中起到重要作用。在转基因(APP/PS1)AD模型小鼠中发现,NLRP3的缺失可显著改善小鼠的空间记忆能力,促进小胶质细胞从促炎M1型向抑炎M2型转变[43]。同时,NLRP3选择性抑制剂MCC950可以显著改善APP/PS1模型小鼠的认知能力,降低小胶质细胞介导的神经炎症,促进Aβ吞噬[44]。因此,NLRP3炎症小体的激活在AD中起到了病情加重的作用,通过促进神经炎症的发生,降低了小胶质细胞细胞对蛋白沉积的清除能力,从而加剧对神经细胞的损伤[45]。
α突触核蛋白可在成体神经干细胞(adult neural stem cells,ANSC)中激活TLR/NF-кB和NLRP3/胱天蛋白酶1信号通路,NLRP3的缺失可减弱α突触核蛋白对ANSC增生的抑制作用[46]。尽管有研究报道,NLRP3在CNS中仅表达在小胶质细胞中而非星形胶质细胞[47],但仍有研究发现,CNS中PD的发生与NLRP3在星形胶质细胞中的激活相关,如在MPTP诱发的PD模型中发现,解偶联蛋白2的缺失,通过激活星形胶质细胞中的NLRP3炎症小体,加剧了多巴胺神经元的丢失[48]。以上研究揭示,NLRP3在PD的发病中可能承担了放大炎症反应,加重神经损伤的作用。上述研究发现,NLR的激活能在减少蛋白沉积清除的同时,通过神经炎症加剧神经损伤。
2.2 精神疾病
大量的临床研究发现,抑郁症和精神分裂症等精神疾病的发生与促炎细胞因子IL-1β和IL-18有显著的相关性[49]。NLRP3和IL-1β的水平在抑郁症模型小鼠的大脑中显著上调[50],说明NLRP3炎症小体在抑郁症发病过程中发挥作用。同时,抗抑郁药物的使用能显著降低患者血清中NLRP3炎症小体组分和促炎细胞因子(IL-1β和IL-18)的含量。NLRP3的缺失或抑制可显著缓解模型小鼠的抑郁样行为[49]。这些结果说明NLR与神经退行性疾病和抑郁症发病的紧密关系,同时意味着对NLR的特异性抑制有可能成为治疗此类疾病的新的治疗方案。
3 视黄酸诱导基因蛋白Ⅰ(retinoic acidinducible gene-Ⅰ,RIG-Ⅰ)样受体
RIG-I样受体(RIG-I-like receptors,RLR)是细胞内一类可识别病毒RNA的免疫识别受体,RLR的激活可引起Ⅰ型IFN的释放[51](图1)。在脊髓损伤发生后6 h内,RIG-I和黑色素瘤分化相关蛋白5(melanoma differentiation-associated protein 5,MDA5)的水平即发生了显著上调,同时伴随IFN-α和IFN-β表达升高[52]。RIG-I和MDA5通过调控星形胶质细胞中神经胶质纤维酸性蛋白和波形蛋白(vimentin)参与CNS外伤后激活星形胶质细胞的过程[52]。线粒体抗病毒信号蛋白(mitochondrial antiviral signaling protein,MAVS)作为RIG-I的联接蛋白,它的激活可促进自噬作用,在小胶质细胞的激活、神经炎症和相关的神经疾病中发挥重要作用。MAVS可通过激活NF-кB和干扰素调节因子3信号通路引起神经炎症[53]。MAVS的缺失可保护MPTP导致的小胶质细胞激活和多巴胺神经元丢失[54]。同时,线粒体钙转运蛋白可通过与MAVS直接相互作用,介导内质网应激引起的炎症反应[55]。另外,MAVS可介导NLRP3炎症小体的线粒体移位,从而调控其激活过程[56],提示MAVS在NLRP3炎症小体激活以及免疫稳态的维持方面起到重要作用。
4 cGAS-STING信号通路
环二核苷酸合成酶(cyclic GMP-AMP synthase,cGAS)是细胞浆中重要的DNA受体。胞浆中游离的DNA片段被视为DNA病毒入侵或细胞损伤的标志,它们可被cGAS识别,从而催化环二核苷酸(cyclic guanosin monophosphate-adenosin monophosphate,cGAMP)的合成。cGAMP作为第二信使,与内质网蛋白干扰素基因刺激蛋白(stimulator of interferon genes,STING)结合并激活下游信号通路[57-59]。近期研究发现,炎症小体的激活可引起胱天蛋白酶1与cGAS的相互作用,被剪切激活的胱天蛋白酶1能切割cGAS,从而抑制cGAS-STING介导的IFN产生[60]。这一发现揭示了cGASSTING信号通路与炎症小体的交叉调节,也暗示了cGAS-STING在神经疾病中发挥重要作用,但目前尚无相关报道。
5 靶向神经炎症药物的研究
神经系统疾病的研究一直以来都是神经科学领域研究的重点,然而目前的临床治疗手段,无论是药物治疗还是手术治疗,都只能达到改善症状的目的,不能有效阻止病情的发展,更无法彻底治愈。以AD为例,目前由美国国家食品与药品监督局(Food and Drug Administration,FDA)批准能用于AD临床治疗的药物仅有5种,其中4种为乙酰胆碱酯酶抑制剂,另外一种是天门冬氨酸受体拮抗剂。它们均能在一定程度上改善AD症状,但通常伴随严重的副作用。其中,他克林(tacrine)是第一种被FDA批准用于AD治疗的药物,在2013年因其肝毒性的报道而被停用[61-65]。在AD中,Aβ为核心的老年斑块和tau蛋白过磷酸化形成的神经原纤维缠结被认为是是导致神经损伤和病情恶化的主要因素。因此,靶向Aβ和tau蛋白的药物研究一直是AD治疗研究的热点,然而大量的研究止步于临床试验阶段,目前仍没有一种药物能在AD临床治疗中发挥显著疗效。在PD中,临床治疗目前主要集中于提高脑内多巴胺含量和改善多巴胺受体功能,其中主要包括多巴类制剂、抑制多巴胺降解的药物和多巴胺受体兴奋剂。2017年3月,FDA新批准了沙芬酰胺(safinamide)用于PD的临床治疗[66]。此药是一种单胺氧化酶B抑制,可以抑制剂多巴胺的降解,增加多巴胺在脑内含量,达到保护多巴胺能神经元的作用。在靶向神经炎症药物的研究中,非甾体抗炎药物(non-steroidal anti-inflammatory drugs,NSAID)用于神经退行性疾病的治疗的研究一直以来是该领域的热点。研究发现,布洛芬(ibuprofen)可有效缓解AD模型小鼠的症状,改善小鼠的认知功能[67]。近期研究发现,一种名为AL7的新型NSAID可有效抑制神经炎症的发生且有可能应用于AD的治疗[68],然而随后的多个研究未发现NSAID使用能显著改善人类或模型动物的神经退行性疾病相关的认知障碍[69-72]。2015年,Coll等[73]发现,NLRP3炎症小体特异性抑制剂MCC950可在体有效减少促炎细胞因子IL-1β的分泌,减轻实验性变态反应性脑脊髓炎模型小鼠的症状,增加冷吡啉相关自发炎症综合症(cryopyrin-associated autoinflammatory syndrom,CAPS)模型小鼠的生存率。随后的研究中发现,MCC950能通过促进Aβ的清除,改善AD模型小鼠(APP/PS1转基因)的认知功能[44]。2017年Jiang等[74]通过药物筛选,发现CY-09能直接与NLRP3的ATP结合位点相互作用,特异性抑制NLRP3炎症小体的活性,同时能缓解CAPS和2型糖尿病模型小鼠的症状。此外,TLR4抑制剂瑞沙托维(resatorvid)在创伤性脑损伤中具有神经保护作用[75-76]。尽管目前仍没有靶向神经炎症的药物被美国FDA批准用于此类神经疾病的临床治疗,但这些研究为能有效缓解,甚至治愈此类神经系统疾病的研究提供新的方向。
6 结语
越来越多的证据显示,许多神经系统疾病的发生发展与神经免疫炎症密切相关,其中先天免疫受体介导的神经炎症发挥重要作用。尽管目前已对先天免疫受体介导的神经炎症的作用以及调控机制有了一定认识,但相较于外周免疫系统活化,脑内神经炎症的活化机制还需要进一步阐明,同时外周免疫系统如何影响神经炎症,它们两者的互作如何影响神经疾病的进程都需要进一步研究。针对目前临床缺乏行之有效的治疗药物,免疫炎症激活机制的阐明,以及靶向神经炎症药物的开发以及应用,为我们提供了一种新的治疗策略和手段。
[1 ]Gendelman HE.Neural immunity:friend or foe?[J].J Neurovirol,2002,8(6):474-479.
[2 ]Ransohoff RM.How neuroinflammation contributes to neurodegeneration[J].Science,2016,353(6301):777-783.
[3 ]Heneka MT,Carson MJ,El Khoury J,Landreth GE,Brosseron F,Feinstein DL,et al.Neuroinflammation in Alzheimer′s disease[J].Lancet Neurol,2015,14(4):388-405.
[4 ]McManus RM,Heneka MT.Role of neuroinflammation in neurodegeneration:new insights[J].Alzheimers Res Ther,2017,9(1):14.
[5 ]Vivekanantham S,Shah S,Dewji R,Dewji A,KhatriC,OlogundeR.Neuroinflammationin Parkinson′s disease:role in neurodegeneration and tissue repair[J].Int J Neurosci,2015,125(10):717-725.
[6 ]Jassam YN,Izzy S,Whalen M,McGavern DB,El Khoury J.Neuroimmunology of traumatic brain injury: time for a paradigm shift[J].Neuron,2017,95(6):1246-1265.
[7]Rossi S,Studer V,Motta C,Polidoro S,Perugini J,Macchiarulo G,et al.Neuroinflammation drives anxiety and depression in relapsing-remitting multiple sclerosis[J].Neurology,2017,89(13):1338-1347.
[8 ]Ramesh G,MacLean AG,Philipp MT.Cytokines and chemokines at the crossroads of neuroinflammation,neurodegeneration,and neuropathic pain[J/OL].Mediators Inflamm,2013,2013:480739(2013-08-12).http://dx.doi.org/10.1155/2013/480739
[9 ]Jack CS,Arbour N,Manusow J,Montgrain V,Blain M,McCrea E,et al.TLR signaling tailors innate immune responses in human microglia and astrocytes[J].J Immunol,2005,175(7):4320-4330.
[10 ]Lafon M,Megret F,Lafage M,Prehaud C.The innate immune facet of brain:human neurons express TLR-3 and sense viral dsRNA[J].J Mol Neurosci,2006,29(3):185-194.
[11 ]Zhang W,Wang LZ,Yu JT,Chi ZF,Tan L.Increased expressions of TLR2 and TLR4 on peripheral blood mononuclear cells from patients with Alzheimer′s disease[J].J Neurol Sci,2012,315(1-2):67-71.
[12 ]Walter S,Letiembre M,Liu Y,Heine H,Penke B,Hao W,et al.Role of the toll-like receptor 4 in neuroinflammation in Alzheimer′s disease[J].Cell Physiol Biochem,2007,20(6):947-956.
[13 ]Letiembre M,Liu Y,Walter S,Hao W,Pfander T,Wrede A,et al.Screening of innate immune receptors in neurodegenerative diseases:a similar pattern[J].Neurobiol Aging,2009,30(5):759-768.
[14 ]Frank S,Copanaki E,Burbach GJ,Müller UC,Deller T.Differential regulation of toll-like receptor mRNAs in amyloid plaque-associated brain tissue of aged APP23 transgenic mice[J].Neurosci Lett,2009,453(1):41-44.
[15 ]Jana M,Palencia CA,Pahan K.Fibrillar amyloidbeta peptides activate microglia via TLR2:implications for Alzheimer′s disease[J].J Immunol,2008,181(10):7254-7262.
[16 ]Richard KL,Filali M,Préfontaine P,Rivest S.Tolllike receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1-42 and delay the cognitive decline in a mouse model of Alzheimer′s disease[J].J Neurosci,2008,28(22):5784-5793.
[17 ]Minoretti P,Gazzaruso C,Vito CD,Emanuele E,Bianchi M,Coen E,et al.Effect of the functional toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer′s disease[J].Neurosci Lett,2006,391(3):147-149.
[18 ]Tahara K,Kim HD,Jin JJ,Maxwell JA,Li L,Fukuchi K.Role of toll-like receptor signalling in a beta uptake and clearance[J].Brain,2006,129(Pt 11):3006-3019.
[19 ]Stewart CR,Stuart LM,Wilkinson K,van Gils JM,Deng J,Halle A,et al.CD36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer[J].Nat Immunol,2010,11(2):155-161.
[20 ]Tang SC, Lathia JD, Selvaraj PK, Jo DG,Mughal MR,Cheng A,et al.Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal[J].Exp Neurol,2008,213(1):114-121.
[21]Kim C,Ho DH,Suk JE,You S,Michael S,Kang J,et al.Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia[J/OL].Nat Commun,2013,4:1562(2013-03-05).http://www.nature.com/articles/ncomms2534
[22 ]Doorn KJ,Moors T,Drukarch B,van de Berg WDj,Lucassen PJ,van Dam AM.Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson′s disease patients[J/OL].Acta Neuropathol Commun,2014,2:90(2014-08-07).http://www.actaneurocomms.org/content/2/1/90
[23 ]Stefanova N,Fellner L,Reindl M,Masliah E,Poewe W,Wenning GK.Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons[J].Am J Pathol,2011,179(2):954-963.
[24 ]Noelker C, Morel L, Lescot T, Osterloh A,Alvarez-Fischer D,Breloer M,et al.Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease[J/OL].Sci Rep,2013,3:1393(2013-03-06).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3589722/pdf/srep01393.pdf
[25 ]Gárate I,García-Bueno B,Madrigal JL,Bravo L,Berrocoso E,Caso JR,et al.Origin and consequences of brain toll-like receptor 4 pathway stimulation in an experimental model of depression[J/OL].J Neuroinflammation,2011,8:151(2011-11-03).http://www.jneuroinflammation.com/content/8/1/151
[26]Jizhong S,Qiaomin W,Chao W,Yanqing L.Corticotropin-releasing factor and toll-like receptor gene expression is associated with low-grade inflammation in irritable bowel syndrome patients with depression[J/OL].Gastroenterol Res Pract, 2016,2016:7394924(2016-07-12).http://dx.doi.org/10.1155/2016/7394924
[27]Wieck A,Grassi-Oliveira R,do Prado CH,Viola TW,Petersen LE,Porto B,et al.Toll-like receptor expression and function in type Ⅰ bipolar disorder[J].Brain Behav Immun,2016,54:110-121.
[28]Oliveira J, Busson M, Etain B, Jamain S,Hamdani N,Boukouaci W,et al.Polymorphism of toll-like receptor 4 gene in bipolar disorder[J].J Affect Disord,2014,152-154:395-402.
[29]Li Y,Li H,Zhang Y,Sun X,Hanley GA,Le Sage G,et al.Toll-like receptor 2 is required for opioidsinduced neuronal apoptosis[J].Biochem Biophys Res Commun,2010,391(1):426-430.
[30 ]Brea D,Blanco M,Ramos-Cabrer P,Moldes O,Arias S,Pérez-Mato M,et al.Toll-like receptors 2 and 4 in ischemic stroke:outcome and therapeutic values[J].J Cereb Blood Flow Metab,2011,31(6):1424-1431.
[31]TangSC, ArumugamTV, XuX, ChengA,Mughal MR,Jo DG,et al.Pivotal role for neuronal toll-like receptors in ischemic brain injury and functional deficits[J].Proc Natl Acad Sci USA,2007,104(34):13798-13803.
[32]Ziegler G,Harhausen D,Schepers C,Hoffmann O,Röhr C,Prinz V,et al.TLR2 has a detrimental role in mouse transient focal cerebral ischemia[J].Biochem Biophys Res Commun,2007,359(3):574-579.
[33]Ziegler G,Freyer D,Harhausen D,Khojasteh U,Nietfeld W,Trendelenburg G.Blocking TLR2 in vivo protects againstaccumulation ofinflammatory cells and neuronal injury in experimental stroke[J].J Cereb Blood Flow Metab,2011,31(2):757-766.
[34 ]Li HY,Hu J,Zhao S,Yuan ZY,Wan HJ,Lei F,et al.Comparative study of the effect of baicalin and its natural analogs on neurons with oxygen and glucose deprivation involving innate immune reaction of TLR2/TNFα[J/OL].J Biomed Biotechnol,2012,2012:267890(2012-03-21).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3321472/pdf/JBB2012-267890.pdf
[35]De Paola M, Mariani A, Bigini P,Peviani M,Ferrara G,Molteni M,et al.Neuroprotective effects of toll-like receptor 4 antagonism in spinal cord cultures and in a mouse model of motor neuron degeneration[J].Mol Med,2012,18(1):971-981.
[36]Jin R,Yang G,Li G.Inflammatory mechanisms in ischemic stroke:role of inflammatory cells[J].J Leukoc Biol,2010,87(5):779-789.
[37 ]Brea D,Sobrino T,Rodríguez-Yáñez M,Ramos-Cabrer P,Agulla J,Rodríguez-González R,et al.Toll-like receptors 7 and 8 expression is associated with poor outcome and greater inflammatory response in acute ischemic stroke[J].Clin Immunol,2011,139(2):193-198.
[38 ]Tang SC,Yeh SJ,Li YI,Wang YC,Baik SH,Santro T,et al.Evidence for a detrimental role of TLR8 in ischemic stroke[J].Exp Neurol,2013,250:341-347.
[39 ]Tan MS,Tan L,Jiang T,Zhu XC,Wang HF,Jia CD,et al.Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer′s disease[J/OL].Cell Death Dis,2014,5(8):e1382(2024-08-21).http://www.nature.com/cddis
[40]Kaushal V,Dye R,Pakavathkumar P,Foveau B,Flores J,Hyman B,et al.Neuronal NLRP1 inflammasome activation of caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated caspase-6 activation[J].Cell Death Differ,2015,22(10):1676-1686.
[41 ]Saresella M,La Rosa F,Piancone F,Zoppis M,Marventano I,Calabrese E,et al.The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer′s disease[J/OL].Mol Neurodegener,2016,11:23(2016-03-03).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4778358/pdf/13024_2016_Article_88.pdf
[42 ]Terrill-Usery SE,Mohan MJ,Nichols MR.Amyloid-β(1-42)protofibrils stimulate a quantum of secreted IL-1β despite significant intracellular IL-1β accumulation in microglia[J].Biochim Biophys Acta,2014,1842(11):2276-2285.
[43]Heneka MT,Kummer MP,Stutz A,Delekate A,Schwartz S,Vieira-Saecker A,et al.NLRP3 is activated in Alzheimer′s disease and contributes to pathology in APP/PS1 mice[J].Nature,2013,493(7434):674-678.
[44]Dempsey C,Rubio Araiz A,Bryson KJ,Finucane O,Larkin C,Mills EL,et al.Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice[J].Brain Behav Immun,2017,61:306-316.
[45]Tan MS,Yu JT,Jiang T,Zhu XC,Tan L.The NLRP3 inflammasome in Alzheimer′s disease[J].Mol Neurobiol,2013,48(3):875-882.
[46]Fan Z,Lu M,Qiao C,Zhou Y,Ding JH,Hu G.MicroRNA-7 enhances subventricular zone neurogenesis by inhibiting NLRP3/Caspase-1 axis in adult neural stem cells[J].Mol Neurobiol,2016,53(10):7057-7069.
[47]Gustin A,Kirchmeyer M,Koncina E,Felten P,Losciuto S,Heurtaux T,et al.NLRP3 Inflammasome is expressed and functional in mouse brain microglia but not in astrocytes[J/OL].PLoS One,2015,10(6):e0130624(2015-06-19).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4474809/pdf/pone.0130624.pdf
[48]Lu M,Sun XL,Qiao C,Liu Y,Ding JH,Hu G.Uncoupling protein 2 deficiency aggravates astrocytic endoplasmic reticulum stress and nod-like receptor protein 3 inflammasome activation[J].Neurobiol Aging,2014,35(2):421-430.
[49]Alcocer-Gómez E,Cordero MD.NLRP3 inflammasome:a new target in major depressive disorder[J].CNS Neurosci Ther,2014,20(3):294-295.
[50]Zhang Y,Liu L,Peng YL,Liu YZ,Wu TY,Shen XL,et al.Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors[J].CNS Neurosci Ther,2014,20(2):119-124.
[51 ]Szabo A,Bene K,Gogolák P,Réthi B,Lányi Á,Jankovich I,et al.RLR-mediated production of interferon-β by a human dendritic cell subset and its role in virus-specific immunity[J].J Leukoc Biol,2012,92(1):159-169.
[52 ]de Rivero Vaccari JP,Minkiewicz J,Wang X,De Rivero Vaccari JC,German R,Marcillo AE,et al.Astrogliosis involves activation of retinoic acidinducible gene-like signaling in the innate immune response after spinal cord injury[J].Glia,2012,60(3):414-421.
[53]Seth RB,Sun L,Ea CK,Chen ZJ.Identification and characterization of MAVS,a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3[J].Cell,2005,122(5):669-682.
[54]Cheng J,Liao Y,Xiao L,Wu R,Zhao S,Chen H,et al.Autophagy regulates MAVS signaling activation in a phosphorylation-dependent manner in microglia[J].Cell Death Differ,2017,24(2):276-287.
[55]Cheng J,Liao Y,Zhou L,Peng S,Chen H,Yuan Z.Amplified RLR signaling activation through an interferon-stimulated gene-endoplasmic reticulum stressmitochondrial calcium uniporter protein loop[J/OL].Sci Rep,2016,6:20158(2016-02-19).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4759556/pdf/srep 20158.pdf
[56 ]Subramanian N,Natarajan K,Clatworthy MR,Wang Z,Germain RN.The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation[J].Cell,2013,153(2):348-361.
[57 ]Sun L,Wu J,Du F,Chen X,Chen ZJ.Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the typeⅠinterferon pathway[J].Science,2013,339(6121):786-791.
[58 ]Wu J,Sun L,Chen X,Du F,Shi H,Chen C,et al.CyclicGMP-AMP isanendogenoussecond messenger in innate immune signaling by cytosolic DNA[J].Science,2013,339(6121):826-830.
[59 ]Zhang X,Shi H,Wu J,Zhang X,Sun L,Chen C,et al.Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING[J].Mol Cell,2013,51(2):226-235.
[60 ]Wang Y,Ning X,Gao P,Wu S,Sha M,Lv M,et al.Inflammasome activation triggers caspase-1-mediated cleavage of cGAS to regulate responses to DNAvirusinfection[J].Immunity,2017,46(3):393-404.
[61 ]Crismon ML.Tacrine:first drug approved for Alzheimer′s disease [J].Ann Pharmacother,1994,28(6):744-751.
[62 ]Pan SY,Guo BF,Zhang Y,Yu Q,Yu ZL,Dong H,et al.Tacrine treatment at high dose suppresses the recognition memory in juvenile and adult mice with attention to hepatotoxicity[J].Basic Clin Pharmacol Toxicol,2011,108(6):421-427.
[63 ]Galisteo M,Rissel M,Sergent O,Chevanne M,Cillard J,Guillouzo A,et al.Hepatotoxicity of tacrine:occurrence of membrane fluidity alterations without involvement of lipid peroxidation[J].J Pharmacol Exp Ther,2000,294(1):160-167.
[64 ]Gutzmann H,Kühl KP,Hadler D,Rapp MA.Safety and efficacy of idebenone versus tacrine in patients with Alzheimer′s disease:results of a randomized,double-blind,parallel-group multicenter study[J].Pharmacopsychiatry,2002,35(1):12-18.
[65 ]Gracon SI,Knapp MJ,Berghoff WG,Pierce M,DeJong R,Lobbestael SJ,et al.Safety of tacrine:clinical trials,treatment IND,and postmarketing experience [J].AlzheimerDisAssocDisord,1998,12(2):93-101.
[66 ]Cruz MP.Xadago(safinamide):A monoamine oxidase B inhibitor for the adjunct treatment of motor symptoms in Parkinson′s disease[J].P T,2017,42(10):622-637.
[67 ]Van Dam D,Coen K,De Deyn PP.Ibuprofen modifies cognitive disease progression in an Alzheimer′s mouse model[J].J Psychopharmacol,2010,24(3):383-388.
[68]Cacciatore I,Marinelli L,Fornasari E,Cerasa LS,Eusepi P,Türkez H,et al.Novel NSAID-derived drugs for the potential treatment of Alzheimer′s disease[J/OL].Int J Mol Sci,2016,17(7):1035(2016-06-30).http://www.mdpi.com/journal/ijms
[69 ]Miguel-Álvarez M,Santos-Lozano A,Sanchis-Gomar F,Fiuza-Luces C,Pareja-Galeano H,Garatachea N,et al.Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer′s disease:a systematic review and meta-analysis of treatment effect[J].Drugs Aging,2015,32(2):139-147.
[70]Jaturapatporn D,Isaac MG,McCleery J,Tabet N.Aspirin,steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer′s disease[J/OL].Cochrane Database Syst Rev,2012,(2):CD006378(2012-02-15).http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD006378.pub2/epdf/abstract
[71]Rees K,Stowe R,Patel S,Ives N,Breen K,Clarke CE,et al.Non-steroidal anti-inflammatory drugs as disease-modifying agents for Parkinson′s disease:evidence from observational studies[J/OL].Cochrane Database Syst Rev,2011,(11):CD008454(2011-11-09).http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008454.pub2/epdf/abstract
[72]Hillmann A,Hahn S,Schilling S,Hoffmann T,Demuth HU,Bulic B,et al.No improvement after chronic ibuprofen treatment in the 5XFAD mouse model of Alzheimer′s disease[J].Neurobiol Aging,2012,33(4):833.e39-833.e50.
[73 ]Coll RC,Robertson AA,Chae JJ,Higgins SC,Muñoz-Planillo R,Inserra MC,et al.A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases[J].Nat Med,2015,21(3):248-255.
[74 ]Jiang H,He H,Chen Y,Huang W,Cheng J,Ye J,et al.Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders[J].J Exp Med,2017,214(11):3219-3238.
[75]Zhang D,Li H,Li T,Zhou M,Hao S,Yan H,et al.TLR4 inhibitor resatorvid provides neuroprotection in experimental traumatic brain injury:implication in the treatment of human brain injury[J].Neurochem Int,2014,75:11-18.
[76 ]Matsunaga N,Tsuchimori N,Matsumoto T,Ii M.TAK-242(resatorvid),a small-molecule inhibitor of Toll-like receptor(TLR)4 signaling,binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules[J].Mol Pharmacol,2011,79(1):34-41.

