New surface finishing technology for the future —Supercritical fluid technology
( Nanjing University, Nanjing 210023, China )
【综述】
New surface finishing technology for the future —Supercritical fluid technology
FANG Jing-li
( Nanjing University, Nanjing 210023, China )
The principles and characteristics of supercritical fluid technology and its application and progress in various aspects of surface finishing were reviewed. The emphasis is put on supercritical CO2cleaning of silicon wafers, supercritical extraction and purification of intermediates for plating and chemical engineering, supercritical fluid preparation of nanoparticles, supercritical fluid electrodeposition and chemical deposition, and supercritical water oxidation in the treatment of various refractory organic wastewater, industrial sludge and waste printed circuit boards. The progress of supercritical fluid processing device was described. The facts show that supercritical fluid technology has broad application prospects in surface finishing. It is a new way to make the old-fashioned surface finishing industry more advanced in technology, more environmentally friendly in process and superior in the quality of coatings.
supercritical fluid; surface finishing; cleaning; electrodeposition; electroless plating; composite plating; oxidation; wastewater treatment
1 Formation of supercritical fluid and its properties[1-5]
1. 1 Supercritical state and supercritical fluid
As temperature and pressure change, pure substance in a closed container will show liquid, gas and solid states. When the temperature and pressure reach a certain critical point, liquid and gas interface will disappear. Liquid and gas merge into a uniform fluid, which is called “supercritical fluid” ( or SCF in short ). The temperature at the critical point is called critical temperature, and the pressure at the point is called critical pressure (SeeFigure 1).Table 1shows the critical point data from a variety of commonly used fluid. Severe changes of the physical and chemical properties of the fluid are seen near the critical point, such as density, viscosity, solubility, heat capacity, diffusion coefficient and dielectric constant ( SeeTable 2andTable 3).

Figure 1 Supercritical state and supercritical fluid[6]图1 超临界状态与超临界流体[6]

Table 1 Critical points of commonly used supercritical fluids[7]表1 常用超临界流体的临界点数据[7]

Table 2 Comparison of the properties of gas, liquid and supercritical fluid[8]表2 气体、液体和超临界流体的性质比较[8]

Table 3 Comparisons of the properties of supercritical CO2and ordinary solvents[7]表3 高密度CO2超临界流体与一般清洁溶剂的特性比较[7]
1. 2 Characteristics of supercritical fluid
Supercritical fluid demonstrates the properties of gas and liquid simultaneously (dual property).
Like a liquid: It is close to liquid in density, solubility, heat transfer coefficient, and hundreds of times larger than those of gas. Because the solubility of a substance is proportional to the density of the solvent, and dielectric constant changes dramatically with pressure, thus supercritical fluid is an excellent solvent that can dissolve many solids such as insoluble resin, oil pollution, pesticides, caffeine, SiN, silicon wafer and residues of printed circuit boards after etching.
Like a gas: It is close to gas in viscosity, surface tension and diffusion coefficient, and two orders of magnitude higher than that of a liquid in diffusion rate. Its transfer rate is far higher than that of a liquid. It can be mixed with most gases and has high compressibility. Change in temperature and pressure can change its density and solubility. It also has strong liquidity, penetration, drilling force and expansion force.
Pressure and temperature change can alter phase transition and change in density.
Supercritical fluid can be recycled, saving resources and cost. There are many types of supercritical fluids. Carbon dioxide and water is the most commonly used ones.
It shows fromTable 1that among many supercritical fluids, carbon dioxide has the lowest critical temperature and critical pressure. It is energy efficient and environmentally friendly. Its raw material is readily available and inexpensive. It has strong ability to dissolve. It is non-toxic, flame retardant, and easy to recycle. Its products are easy to purify. It is suitable for mass production and application. As a result, it has become the most widely used supercritical fluid in our country and abroad.
As it shows fromTable 2andTable 3, the density of supercritical fluid is close to liquid. As a result, its solvation ability is very strong. Because its viscosity is close to a liquid, its diffusion capacity is 100 times larger that that of a liquid. Its number of hydrogen bond decreases from 1.93 to 0.7 and below. Therefore it has very strong solvation ability, excellent liquidity and transitivity. It is a good pollution-free green solvent which can replace toxic and volatile organic solvent, eliminating the pollution to the environment by organic solvents.
2 Application of supercritical fluid in surface finishing field
2. 1Application of supercritical CO2in cleaning of printed circuit boards and microelectronic parts[9-11]
At a constant temperature, the solubility of the supercritical fluid substance increases with increasing pressure. The solubility can be varied within the range of 100-1000 fold by adjusting temperature and pressure. This feature of supercritical fluid will maximize solubility of the target (for example contaminants to be removed) in the supercritical fluid, and improve operational efficiency. On the other hand, through decreasing pressure and/or temperature, the target and supercritical fluid will be easily separated. In addition, the liquidity and transitivity of supercritical fluid are close to gas, which makes the migration of the target in supercritical fluid very fast. This will expedite the process and improve production efficiency. Currently the applications of supercritical fluid in cleaning include:
(1) Electronics: printed circuit boards, silicon wafers and other microelectronic devices.
(2) Defense industry: instrument bearings, aerospace components, etc.
(3) Optical industry: laser lenses, contact lenses, fiber optical components, etc.
(4) Precision machinery: precision bearings, micro drive assembly, and fuel nozzle.
(5) Medical devices: pacemakers, blood dialysis tube, surgical appliances, etc. (sterilization and cleaning).
(6) Food Industry: edible rice (removal of pesticides, killing bacteria and eggs of worms).
Cleaning of semiconductor nano- and micro-devices, and many high-end products using supercritical fluid has been funded by the National Natural Science Foundation of China. Some research results will be obtained recently.
Currently there are two main ways of cleaning using supercritical fluid: clearance cleaning and semicontinuous cleaning. The process of flow is shown below:

The advantages of supercritical CO2cleaning technology:
(1) Supercritical CO2has very low surface tension and viscosity, and very high diffusion. It penetrates into deep hole and slit easily. Thus it has very good cleaning effect. It is the most advanced and environmentally friendly new cleaning technology.
(2) CO2is non-flammable, non-toxic, with good chemical stability. It is easily separated, and does not produce side effects.
(3) CO2comes from chemical byproducts. It is cheap and readily available, and can be recycled easily. It can reduce the emission of greenhouse gases.
(4) The product after supercritical CO2treatment does not need to be dried. It does not have solvent residue, which simplifies the separation and post-treatment step of the solvent with no solvent waste and wastewater.
(5) Supercritical CO2has high efficiency for the removal of various pollutants, and low solvent and energy consumption. It can replace varieties of toxic organic solvents, and is environmentally friendly.
(6) The solubility of supercritical CO2can be adjusted by the pressure of the fluid.
The drawbacks of supercritical CO2cleaning technology:
(1) Supercritical CO2cleaning system requires high pressure. The equipment cost is high.
(2) It is suitable for high-volume and high-end products. For low-end product, the cost is relatively high.
Figure 2is the effect of supercritical CO2(also called as SCCO2in short) cleaning silicon wafer.

Figure 2 Cleaning effect of SCCO2on silicon wafer图2 SCCO2清洗硅晶圆的效果
2. 2Application of supercritical extraction technology in the purification of electroplating intermediates
In the supercritical state, the solubility of the solute will vary with temperature and pressure, which makes it possible for the products and reactants to be removed from the mixture successively and respectively. This facilitates the separation of the product, the reactants, the byproducts and the catalysts. Supercritical fluid may selectively dissolve some components from a mixture. Then separate them under reduced pressure, increased temperature or using adsorption. This chemical separation method is called supercritical fluid extraction. In recent years, the method has seen many applications in the extraction of active ingredients from the plants used for medicine, the active pharmaceutical ingredient in a medicine. It is also used in the refining and purification of various additives and cosmetics. Supercritical chemical reaction can increase the rate of chemical reaction, improve mass transfer, reduce the reaction temperature, increase the conversion of the reactants and enhance the selectivity of the products. The separation of the products is also very easy.
Surface finishing additives and complexing agent is a large class of chemicals. There are many needs of chemical synthesis, and the products are usually mixtures which need further separation and purification. In order to make stable high quality plating additive, the purity of additives needs to improve, various harmful impurities need to be removed[12-13].
China has a lot of factories that make intermediates of electroplating additive. Due to the difficulty in the separation and purification of the products, many factories often sell the products without separation and purification. Although the price is relatively cheap, they can only be used to make low-end products of low or unstable quality. The most commonly used method of separation and purification is to extract with water and then precipitate with ethanol, or extract with an alcohol and then precipitate with water. This method requires large amounts of organic solvent, and the process is complicated and time-consuming. In addition, there are residuals of organic solvents.
The critical temperature of supercritical CO2is only 31.06oC, which is close to room temperature. The critical pressure is only 7.39 MPa. It is possible to extract the electroplating intermediates of high boiling points and low volatility under mild condition, so they can be further separated and purified. Nonpolar supercritical CO2has high solubility in lipophilic non-polar organic intermediates. They can be separated and purified by adjusting the temperature and pressure during extraction. So the purity of the organic intermediates is greatly improved. This is the key for the development of our electroplating intermediates industry to high-end.
2. 3Plating of high corrosion resistance coatings using nanoparticles prepared by supercritical fluid[14]
A necessary condition for metal corrosion reactions is that the electrochemical corrosion must occur in an aqueous medium. If water is blocked from the metal surface, the corrosion is difficult to occur. Recently some foreign countries proposed a new theory of using “superhydrophobic surface to inhibit corrosion”, which provided new ideas and approaches for the development of long-term and intelligent anticorrosion.
From the superhydrophobic surface of the lotus leaf, people realize that in order to get superhydrophobic surface, the roughness of the metal surface has to be at micro-nanometer level. Since the size of water droplets has more than a micro-nano, they cannot enter the depression of metal surface, so chemical corrosion will not happen. Secondly, if a hydrophobic complex film is formed at the micro-nano surface layer to prevent water from contacting with the micro-nano surface, it will be even harder for corrosion to happen.
The easiest way to convert a metal coating into a micro-nano structure is to add nanoscale particles (such as nanoscale TiO2, Al2O3, ZrO2, etc.) to the normal plating bath, using composite plating method to make nanoparticles inlaid into coating. This not only changes the coating roughness to a micro-nanostructures, but also make the coating denser and lower its porosity, so that not only the corrosion resistance of the coating, but also its hardness and wear resistance are improved.
Ishizaki et al[15]obtained nanometer conversion coating of magnesium alloy from the conversion coating bath of cerium oxide nanoparticles. They also formed a hydrophobic film from fluorosilanes which has superhydrophobic structure. The contact angle of water reaches 153.2°. After soaking in 5% NaCl solution for 24 h, low frequency impedance modulus of the magnesium alloy after superhydrophobic treatment is 5 magnitude above that of the untreated magnesium alloy. Polarization curves also show that the corrosion current of magnesium alloy with the superhydrophobic treatment is much smaller than that of the untreated magnesium alloy.
Yanqing Guo et al[8]added nano Al2O3powder to pyrophosphate system Cu–Sn alloy plating bath. When the concentration of nano-Al2O3was 8 g/L and pH = 9 , the microstructure of Cu–Sn alloy deposit had significant change. The Al content in the coating reached 10.72% (by mass), and more uniform distribution of nano-Al2O3was found. With the increase of the concentration of nano-Al2O3, the corrosion potential of Cu–Sn alloy coating was shifted by 0.3303 V. The corrosion current was three orders of magnitude lower than the one without nano-Al2O3.The microhardness, corrosion resistance, abrasion resistance of the coating, and its bonding strength with substrate all improved significantly.
In the past nano-coating powder materials were mostly prepared by spraying and drying, fine grinding, and crystallization from supersaturated solution. But the size of the particles, uniformity, mobility, etc. can hardly meet the required technical standards of many industries. So searching for high purity crystalline, uniform size, good mobility of nanoparticles is the current hot topic. Using supercritical fluid approach in the preparation of nanoparticles is the latest and most advanced manufacturing methods internationally. Supercritical fluid solvents have a lot of important features that many solvents do not, such as rapid change of density, solvation ability, viscosity, dielectric constant, and diffusion coefficient with the change of temperature and pressure. This means that its property can be adjusted continuously with the change of the pressure without changing the chemical composition. When supercritical liquid expands rapidly from a supercritical state to the low pressure and low temperature gas state, the solubility of the solute sharply decline, which brought rapid nucleation, growth of solute particles and deposition. The resulting particle size distribution can be adjusted by the pressure, temperature, aperture size of fluid ejection nozzle and spraying speed. It is easy to obtain a superfine powder of 1-1000 nm. This process is carried out in a quasi-uniform medium and the precipitation process can be better controlled. It is a promising new technology[10].
2. 4Preparation of nanometer chemical plating and nanocomposite coatings by supercritical fluid deposition
Supercritical fluid deposition (SCFD) is a new technology developed in recent years abroad for preparing high-quality metallic coating and metal nanoparticles. The technology uses supercritical fluid as a media to obtain metal coatings, metal-matrix nanocomposite coatings and nano-metal catalyst after reduction of metal compounds. Because the supercritical fluid does not have gas-liquid interface, there is no surface tension. The diffusion rate of the medium in it is equivalent to the diffusion velocity of the gas. As a result high dispersion capability, high coverage, high purity, low resistance, and strong substrate binding, uniform particle size and controllable coating, granules, film, rod or wire can be obtained on the surface of semiconductor wafer, metal, polymer, porous material, and many inorganic materials. It has broad application prospect in microelectronics, catalysis and surface finishing field.
Watkins et al[16]reduced organometallic compounds with hydrogen in supercritical CO2environment. They managed to deposit Cu, Ni, Pt, Au, Pd, Ru and other metal coating on the unmodified silicon oxide wafer and silicon wafer with TaN and Kapton coating. The metal coating can not only deposited on a flat surface of the base metal, but also on the surface of a fine pattern substrate with a conformal coverage of the metal coating. In microelectronics field, copper has replaced aluminum as the preferred inline metal. This is because of the low resistance and excellent electromigration resistance of copper. However, it is very difficult to make a fine pattern using etching method. Because so far there is no suitable precision dry etching process. Seed layer puttering is used followed by Damascene process, which electroplates a copper layer into the trenches and vias. When the size of vias is less than 45 nm, it is very difficult to obtain a continuous and conformal copper seed layer on a high-aspect-ratio feature by sputtering method. The high diffusivity, low viscosity and zero surface tension of supercritical fluid solves the problems of sputtering method. Highly conformal and high filling power of copper seed layer can be obtained[17].
Zhao Bin et al[18]obtained copper seed layer in supercritical CO2with Ru substrate, 0.75 mol/L hydrogen as the reducing agent, 0.002 mol/L bis(2,2,6,6-tetramethyl-3,5-hexanedionato)copper as copper salt. The resulting copper layer is very smooth, with thickness of 20 nm and RMS roughness of 6.1 nm. It is suitable to be used as inline copper seed layer in ultra large scale integrated circuit (ULSI).Figure 3is the photos of SCF nanometer copper layer deposited on a silicon substrate.
Wang Yanlei et al[19]obtained metal palladium plating layer on a single crystalline silicon using supercritical CO2as solvent, hexafluoroacetylacetonato palladium [Pd(II)(hfac)2] as palladium salt, at a temperature of 100 °C, under the of pressure 12-18 MPa, and after hydrogen catalytic reduction. The coating is uniform and continuous with thickness of 0.3-1.5 μm. It has the crystal structure of metal palladium. The grain size changes with pressure. At pressure of 12, 15 and 18 MPa, the grain size is 30-60 nm, 90-120 nm and 150-180 nm, respectively. This indicates that when the temperature is constant, the greater the pressure, the greater the grain size. On a cylindrical cavity base, a deposition of a uniform palladium layer inside and outside of the cavity was obtained. This method has broken through the limitations of the traditional method which can only prepare plane deposition. It is a low carbon and environmentally friendly coating method. Metal Pd has wide application especially in the field of microelectronic industry in which it can be used as connection material. This method should make it more easily to deposited metal coating on complex surfaces or in cavity.Figure 4is the palladium film deposited on a featured silicon testing wafer.
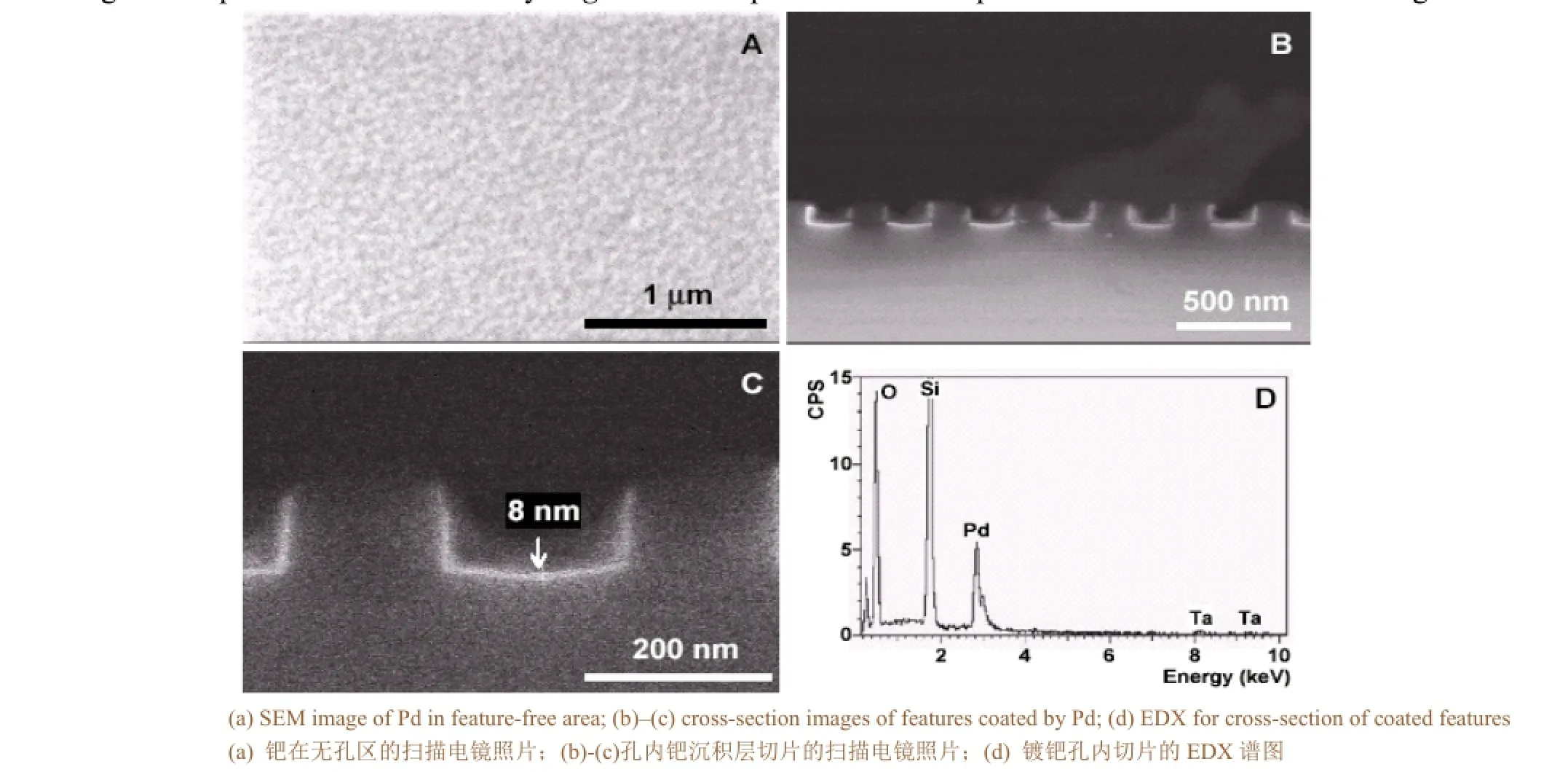
Figure 4 Pd film deposited on a featured silicon testing wafer图4 SCF法在硅基体上沉积纳米钯层
Sun et al[20]obtained nanoparticles of palladium and ruthenium using inorganic PdCl2or RuCl3·3H2O as metal source, supercritical CO2as solvent and methanol as the reducing agent and co-solvent. These nanoparticles obtained on the outer wall of the carbon nanotubes by supercritical deposition dispersed evenly, with high crystallinity and uniform particle size. They are more uniform and more difficult to agglomerate than the Pd, Ru nanoparticles prepared by hydrothermal method.
Watanabe et al[21]studied the deposition of Pt–Ru alloy nanoparticles on Ketjen black (KB) and multiwalled carbon nanotubes at temperature of 120-180 °C, deposition time of 30-90 min, using hydrogen to reduce two metal complexes [Pt(hfac)2] and [Ru(Cp)2] (bis(cyclopentadienyl)-Ru) at the same time. The particle size is about 2 nm, with most alloy particle size of 5 nm or less and the average particle size of 3 nm. It was determined by the test thatthe Pt particles have catalytic effect on the pyrolysis of [Ru(Cp)2] ,while Ru particles catalytic effect on the pyrolysis of [Pt(hfac)2]. This means Pt and Ru have synergistic catalytic effect.
2. 5Applications of supercritical fluid deposition techniques on electroplating[5]
Electroplating technology is to reduce complex metal ions to metal atoms using direct cathodic current, and to form a coating on the substrate surface. Because of the complexity of the structure of the metal matrix, current density varies widely in different parts. The coating is thicker where the current density is larger (such as the edge of the tip portion), while the coating is thinner where the current density is smaller (such as central or deep within the hole. It is difficult to obtain a uniform coverage. This is the reason why conventional plating has poor covering powver and throwing power. And with the extension of plating time, the grain size of the plating will become larger, and the coating may even crack. Usually this can only be improved by adding additional external means such as adjusting the current, temperature, agitation, spraying and hieroglyphic anode[12-13]. It is difficult to obtain uniform coverage of the coating by physical vapor deposition (PVD) in the space. Theoretically uniform deposition can be obtained by chemical vapor deposition (CVD). But due to the volatility of the metal salt, the concentration is very low in the gas phase, and the speed of mass transmission is slow. As a result, ideal coatings still cannot be obtained with this method.
Supercritical fluid is the fluid in a special state which is formed with its temperature and pressure above the critical point. It has low viscosity, controllable density, large diffusion coefficient, zero surface tension, excellent fluidity and permeability, and fast transmission speed. Thus by supercritical fluid electrodeposition, coatings can be obtained with finer grains, smoother surface, better throwing power and covering power, and higher microhardness, wear resistance and corrosion resistance.
The operating temperature of supercritical CO2is 31 °C, and the pressure is 7.3 MPa. It is non-toxic, harmless, inert, cheap, and easily to be recycled. It is an environmentally friendly supercritical fluid.
Tso-Fu Mark Chang et al[22]studied supercritical plating of thin (<100 nm) layer of nickel and found that when plating in supercritical CO2emulsion, as the current density reached 1 A/dm2and time reached 30 s, a complete coverage of a uniform and defect-free nickel layer was obtained. Its grain size was less than 100 nm, and the coating hardness was up 6.951 MPa. While under the same conditions, nickel layer obtained by ordinary plating is uneven with many defects. Its hardness is 30% lower than that of the supercritical plating.
Hikayu Kinashi[23]conducted copper plating experiment with supercritical CO2emulsion. The grain size of the resulting copper layer was only 0.1 μm, and the strength of the copper layer was 6300 MPa, while the grain size of the conventional copper plating layer is 1.0 μm, and the intensity is much smaller.
2. 6Application of supercritical fluid deposition technology in the composite electroforming
Electroforming technology has important applications in computer systems, precision molds and aerospace fields. Performance of electroformed layer has a great impact on the intrinsic quality of the molded part. As mentioned earlier, the nanocomposite plating layer or electroformed layer has better corrosion resistance, abrasion resistance and higher resistance to high-temperature oxidation than ordinary plating layer. Because of its high surface activity, nanoparticles can easily agglomerate in the electrodeposition process which affects the performance of nanocomposite coating.
Supercritical fluid has a liquid-like density, strong solubility characteristics, and a viscosity close to that of a gas. Its dispersion characteristics are between the gas and the liquid, with excellent diffusion properties[24]. Therefore, when composite electroforming is conducted under supercritical fluid conditions, the agglomeration of nanoparticles in the electroforming liquid can be effectively controlled. Nanocomposite particles can be more uniformly distributed in the electroformed layer. The dispersion strengthening effect of the electroformed layer can be increased, and themicrohardness and wear resistance of the electroformed layer can be raised.
Liqin Liu et al[25]researched electroformed nickel with supercritical CO2as a carrier, nano-Al2O3as an additive. The composition and conditions of the electroforming liquid are as follows: NiSO4.6H2O 300 g/L, NiCl2.6H2O 60 g/L, H3BO340 g/L, Al2O3(diameter 30 nm) 60 g/L, polyethylene glycol trimethylnonyl ether 1.2 g/L, pH 4.2-5.5, cathodic current density 4 A/dm2, temperature 40 °C, pressure 14 MPa, magnetic stirrig rate 314 r/min, and time 1 h.
The results showed that microhardness of the composite electroformed layer was 1230 HV, which is several times higher than that of ordinary composite electroformed layer (350.7 HV). Al2O3content of the composite electroforming layer was 9.88%, which has a substantial increase compared with ordinary composite electroforming layer. The Al2O3in the supercritical composite plating layer is very uniform, with small particle size. The agglomeration is not substantial. But under traditional conditions, Al2O3in the composite electroformed layer has poor dispersion, with certain degrees of agglomeration. This is mainly because the supercritical fluid has a low viscosity. It can effectively reduce the surface energy of the nanoparticle and act as a wetting agent to avoid colliding between the particles, which inhibits the agglomeration of nanoparticles and improves the dispersion effect .
3 Application of supercritical fluid technology in electroplating wastewater treatment
The application of supercritical fluid technology in the electroplating wastewater treatment is mainly using supercritical water as a reaction medium, with oxygen, ozone, H2O2, etc. as oxidant. Under the oxidation reaction in a supercritical state, the dehydration reaction and the cleavage reaction, the organic compounds in the waste water, solid waste and sludge are decomposed into CO2, H2O, N2and inorganic salts. Critical temperature of water is 374.3 °C, and the critical pressure is 22.05 MPa. Water is incompressible under normal circumstances. However, supercritical water is a compressible fluid. Its density is close to that of liquid, and its viscosity close to that of gas. Its diffusion coefficient is about 100 times as that of liquid. Therefore supercritical water has both the solubility of liquid, and transmission property of gas. Its dielectric constant is small, much like a non-polar solvent. The oxidation rate of supercritical water increases with the increase of temperature and pressure . More than 99% of organic matter can be removed in a few minutes. If the content of the organic matter is more than 2% in supercritical water, no external heat supply from outside is needed. Therefore it is a novel, efficient, fast, and environmentally friendly new oxidation technology for the removal of the organic compounds that are hard to degrade. It can remove a variety of organic contaminants, and has broad application prospects.
3. 1Supercritical water treatment of wastewater containing S
Most plating additives are organic matters that contain sulfur. Botao Xiang et al[26]studied the process of sulfur-containing waste water treatment using the supercritical water oxidation. When S2-was 58 mg / L, temperature was 450 ℃, pressure was 26 MPa, O/S ratio was 3.47, and the reaction time was 17 s, S2-can be completely oxidized toIncreasing the reaction time, pressure, and O/S ratio could significantly increase the sulfur removal rate.
3. 2Supercritical water treatment of wastewater containing N
Nitrogen-containing organic compounds, such as urea, thiourea, triethanolamine, polyethylenepolyamine, epoxy-amine condensates are often used in plating solutions and the like. These N-containing organic compounds are difficult to be removed by conventional oxidizing agents. But they can be easily removed using supercritical water. Tao Wang et al[27]conducted tests using urea solution as simulated wastewater which contains nitrogen in a continuous flow supercritical water oxidation device. At 400-500 °C, pressure of 24-30 MPa, and reaction time more than 2 min, 95% or more nitrogen-containing organic compounds were removed. As the reaction temperature, pressure and reaction time increase, the removal rate of organic compounds can be significantly increased.
3. 3Supercritical water oxidation treatment of other organic wastewater
Steven. R. Rice and Richard R. Steeper[28]researched the treatment of cyanide, methanol, nitrobenzene, urea,dioxins, polychlorinated biphenyls (PCBs), etc. by supercritical water oxidation technology. They found for most compounds, at temperature of 550 °C and above, residence time of 20 s, the removal efficiency of total organic carbon (TOC) could be 99.95% or more. Ivette Vera Pérez et al[29]treated phenol and dinitrophenol with supercritical water at pressure of 25 MPa and temperature of about 500 °C, the removal rate of TOC was 99.77% after processing for 40 s. Aki et al[30]studied the catalytic supercritical water oxidation of pyridine, using Pt/γ-Al2O3as catalyst. At 24.2 MPa, when the concentration of pyridine was 0.185 mol/L, the concentration of oxygen was 0.1 mol/L, the temperature was 400 °C, the removal rate of pyridine was 95%. The gas-phase products were CO2and N2O. This proves that addition of the catalyst is able to accelerate the rate of the oxidation reaction, improve removal rate of pyridine, and make the reaction conditions milder. As for researches on new highly efficient catalysts, the precious metals and their oxides, transition metal oxides and metal salts, heteropolyacids and carbonyl catalyst have studied. But they all have their own advantages and disadvantages. Good quality and cheap catalysts have not yet been found. It is worthy of further study[31].
3. 4Supercritical water oxidation treatment of industrial sludge
Sludge will be produced from various wastewater treatment, and it is much more difficult to treat sludge than the waste water. This has become the focus and challenges of water treatment. Landfill, burning and pyrolysis are the common methods to treat sludge. Sludge landfill is unable to kill the bacteria, which can contaminate groundwater, and also causes harmful infectious diseases caused by microorganisms. Landfill occupies large amount of land, which is becoming more difficult to find now. Burning will produce large amounts of harmful gases and dust, and also dioxins and other contaminants that are very harmful. Pyrolysis will produce secondary pollutants such as oil. Treating sludge with supercritical water oxidation can avoid these problems. It also has less drain, and no burning of gas and ash. The process is short, and the devices are simple with no secondary pollution. At a temperature of 370-650 °C, under pressure of 22-26 MPa, the sludge treatment rate can reach 99.8%. The final products are CO2, N2and water without NOxand SO2. Therefore there is no secondary pollution, no hazards materials to the environment. It is an environmentally friendly approach.
In 1996, Hydro-Processing company treated municipal and industrial sludges using supercritical water oxidation. They used a tubular reactor system of 90 L/h, and achieved good results. A. Shanableh[32]studied supercritical water oxidation treatment of biological sludge. The results showed that more than 99% of COD (chemical oxygen demand) could be oxidized to a colorless and odorless CO2, H2O, and inorganic salts within 5 min. Motonbu Goto et al[33]treated the sludge from the waste water treatment plant using supercritical water oxidation technology. They used H2O2as the oxidant and the products were colorless and odorless liquid. With the increase of temperature and amount of oxidant, water TOC was significantly reduced.Table 4shows a comparison of supercritical water oxidation (SCWO) and conventional burning and wet air oxidation (WAO). It can be seen fromTable 4that, in terms of travel time and removal rate, or the applicability and follow-up treatment, supercritical water oxidation are superior to conventional wet oxidation and burning method.
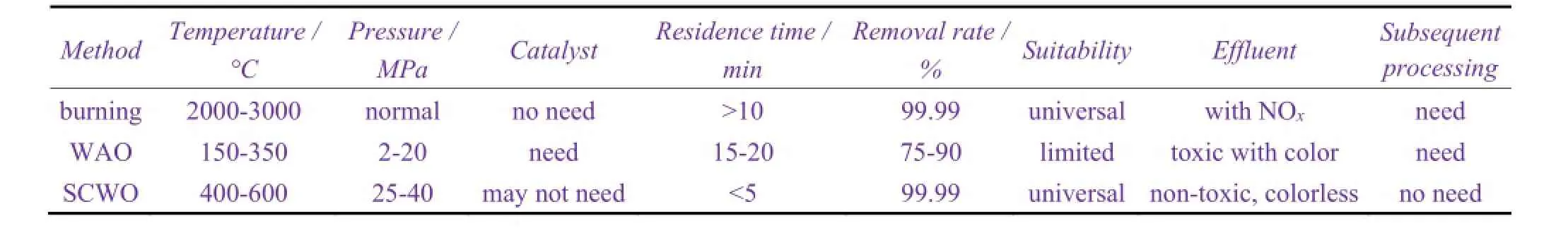
Table 4 Comparison between supercritical water oxidation, wet air oxidation and incineration methods[34]表4 超临界水氧化法与湿式空气氧化法和焚烧法的比较[34]
So far at home and abroad, supercritical water oxidation treatment studies have been carried out for many compounds, including alcohols, acetic acid, phenol, pyridine, PCBs, dioxins, halogenated aromatic compounds,halogenated aliphatic compounds, nitro benzene, urea, DDT, chemical weapons, propellants, etc[35]. The results showed that these organic compounds were almost completely oxidized to CO2, H2O, N2, etc. after the treatment. It has unique advantages especially in the treatment of toxic and hazardous waste which causes difficulty for conventional methods.
3. 5Supercritical water oxidation treatment of waste printed circuit boards
One of the most important application of surface finishing technology is in printed circuit board (PCB), which is the most important part of the electrical and electronic products. PCB has a layered structure made with metal layer (mainly copper foil), reinforcing layer (glass fibers) and adhesive layer (resin). The layers are bonded together by an adhesive layer in between.Table 5lists the composition ratio of the main components of PCB.
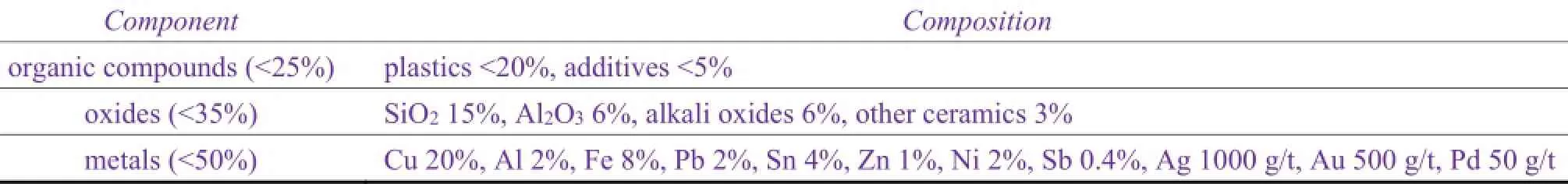
Table 5 Compositions of main parts of PCB[36]表5 PCB中主要成分的组成比例[36]
As seen fromTable 5, PCB contains about 50% of the metal, among which the copper content is up to 20%, much higher than the copper in copper mines. Thus its recycle value is also much greater. Currently PCB recycling technologies include burning, pyrolysis, mechanical and physical methods, chemical treatment and supercritical fluid method. Burning is to break the circuit boards and burn them in the incinerator. The combustible components will decompose, and the metal-rich group and insoluble substances are collected, crushed and recycled by the metal smelter. The advantage of this method is that the process is simple and not time-consuming. It also reduces the volume of the circuit boards. The disadvantage of this method is that it has high energy consumption, and the organic matters cannot be recycled. It emits gas pollution to the environment, and it may produce other toxic substances such as hydrogen bromide and dioxins. In pyrolysis, broken circuit boards are placed in a reactor under an inert gas, heated to a certain temperature till they pyrolysis. Organic compounds becomes hydrocarbons of lower molecular weight and discharged from the reactor in the form of gas. Then they are condensed, cleaned and purified to make fuel or chemical raw materials. The remaining solid residue is a mixture of metal-rich collective, ceramic and glass fibers. After crushing and separation, metals and nonmetallic materials are recycled. The advantage of this method is that organics and metals can both be recycled with high recovery rate, and low secondary pollution. Its disadvantages are high processing temperature, long time, large energy consumption, and more complicated process. Chemical method can be divided into pickling and electrolysis. In pickling, the metal from the crushed board is dissolved with nitric acid, sulfuric acid or aqua regia. After separation, the metal is reduced or electrolysised into a single metal for recycling. The rest of the high concentration copper solution can be recycled as sulfate or electrolysis copper[31]. The advantage of this method is that the polymers and various metals can be separated and recycled with high efficiency, low energy consumption and low cost. Its drawback is that the process will produce large amount of waste water and emissions which are pollutions to environment. Mechanical and physical methods are to crush or pulverize the blank board that has been pretreated. The board particles are fed into the sorting device to be sorted. Finally the collected metallic and nonmetallic mixture is separated and purified. The advantages of mechanical and physical method are simple process and low cost. The recycling process does not require adding chemical solvents, which has small impact on the environment. Its drawback is that the circuit board needs to be pretreated into blank board. What is recycled is semi-finished metal-rich collective, which needs to be furthrer separated and purified by metallurgy. The recovery efficiency is low[37].
Supercritical CO2has similar density and solubility of those of the liquid. It has good liquidity, and diffusioncoefficient and low viscosity similar to those of gas. It can break the adhesive layer in the printed circuit board, so that layers are completely separated in the circuit board and finally metal and glass fiber can be obtained[38]. The process of recycling printed circuit board using supercritical CO2is very simple. First, components on the board are removed. The blank board is cleaned with water and placed into a closed reactor. Then water is added and heated to 280 °C, and the pressure is increased to 40 MPa, making CO2supercritical state. After 4 hours of reaction, air switch is opened. When the vessel is cooled, the container is opened, and separated copper and glass fiber can be obtained.
CO2can be recycled after the exhaust is treated. A comparison of supercritical CO2method and other methods is shown inTable 6. The main advantages of this method are: high recovery rate of the materials; copper foil and glass fiber can maintain their original shape; the process is simple; the whole board can be directly processed without crushing; it is environmentally friendly; there are no harmful gases or waste water, and CO2can be recycled; nnergy and resources consumption are low; and the cost of the recycling process is low.

Table 6 Comparison of five methods for reclamation of printed circuit boards[39]表6 5种印刷电路板回收方法的比较[39]
4 Processing equipment of supercritical fluid
After decades of development, the equipment of supercritical fluid processing technology has been developed from homemade laboratory equipment to pilot equipment. Now many large-scale production equipment has been built in the world and commercial operation has begun. In the United States, supercritical CO2extraction technology is widely used in the extractions of North American palm fruit, cards hair root,Hypericum erectumplant, ginkgo, etc. The supplements made with this method are in short supply. Now supercritical extraction equipment has dedicated large equipment supply at home and abroad. Supercritical water oxidation equipment requires high temperature and high pressure. It is very difficult to manufacture the equipment. In 1994, EWT company of the United States built and put into operation the world’s first supercritical water oxidation plant for Huntsman company in Texas, Austin, to treat long-chain organic compounds and glue. The TOC was more than 50 g/L.This device uses a tubular reactor of 200 m in length, with operating temperature 540-600 °C, pressure 25-28 MPa, and feed rate 1100 kg/h. After the reaction, the removal rate of TOC in the water was more than 99.988%. NOxin the exhaust gas was 0.6 × 10-4, CO was 60 × 10-6, CH4was 200 × 10-6, SO2was 0.12 × 10-6, and ammonia was less than 1 × 10-6. All emissions were direct in line with the local standards. The cost of treating waste using this equipment is only one third of that if using burning[40].
According to the reports, the manufacturers of supercritical water oxidation equipment in our country include Sanmenxia HD Environmental Protection Technology Co., Ltd., Shijiazhuang Development Zone QiLi Technology Co., Ltd. and Nantong HuaAn Supercritical Extraction Company. Sanmenxia HD Environmental Protection Technology Co.. Ltd. began to engage in supercritical water oxidation wastewater effluent treatment since 1998. They did small scale test on pesticide wastewater, papermaking wastewater, chemical wastewater, and pharmaceutical wastewater. The pilot equipment of supercritical water oxidation made by this company has a daily processing capacity of 300 L/d[41]. Chengyu Ma et al[42]and Shijiazhuang Development Zone QiLi Technology Co., Ltd. jointly developed a pilot equipment for near industrious operation. It has a continuous water treatment rate of900-1000 L/d, organic contaminants treatment capacity (COD) of 50000 mg/L or more, and flow rate of highconcentration chemical waste liquor less than 5 m3/d.
5 Conclusion
In the supercritical state, ordinary CO2and H2O become a supercritical fluid, which has many unique physical and chemical properties. It has the dual nature and advantages of liquids and gases. For example, in a supercritical state water is miscible in any proportion with organic matter, oxygen, and air. The gas-liquid interface disappears, and heterogeneous reaction is converted to a faster single-phase reaction. Usually it only takes a few seconds to a few minutes to convert organic pollutant into CO2, H2O, and inorganic salts, which achieves safe disposal of toxic and hazardous materials. The United States has placed it as the most promising waste treatment technology for energy and environment.
Supercritical CO2fluid has a liquid-like density, solubility and good mobility, and also has a diffusion coefficient, drilling force and a low viscosity similar to a gas. It can replace traditional toxic and volatile organic solvents for the extraction and purification of Chinese herbal medicine, medical chemicals, electroplating and chemical plating intermediates,The rapid expansion of supercritical CO2can also be used to prepare nanoparticles that have uniform particle size, controllable thickness and good mobility. Currently this is the most important method for producing nanoparticles.
Supercritical CO2has high solubility to the adhesive material from the waste printed circuit board. It can directly decompose multilayer circuit boards rapidly into copper foil and plastic sheeting, which makes it the simplest and the most environmentally friendly new technology of recycling waste printed circuit board.When used in electrodeposition of finer grain, smoother surface, better covering and throwing power, higher hardness, bette wear and corrosion resistance can be obtained by using supercritical CO2. It has become the preferred new technologies for electroplating, electroless plating, composite plating and electroforming.
Supercritical fluid technology has been widely used in many areas of electronic appliances, precision machinery, optical industry, food industry, pharmaceutical industry, medical equipment, chemical industry and defense industry. But its applications in the field of surface finishing has just started in limited area in a very small scale.I hope that our researchers get to know more about the applications of supercritical fluid in surface finishing in our country and abroad. And more people would carry out research and application on supercritical deposition and waste management, so that our surface finishing industry will be more advanced in technology, more environmentally friendly in process, and better in quality, and move in the direction that really makes “Made in China” become “Created in China”.
Reference:
[1] YE X R, LIN Y H, WANG C M , et al. Supercritical fluid synthesis and characterization of catalytic metal nanoparticles on carbon nanotubes [J]. Journal of Materials Chemistry, 2004,14 (5): 908-913.
[2] LI S F, WU X W, HOU C X, et al. Supercritical fluid technology and application [J]. Modern Chemical Industry, 2007, 27 (2): 1-7, 9.李淑芬, 吴希文, 侯彩霞, 等. 超临界流体技术开发应用现状和前景展望[J]. 现代化工, 2007, 27 (2): 1-7, 9.
[3] YIN J Z, LIU X, DING X W. Research progress of supercritical fluid technology [J]. Jiangsu Chemical Industry, 2002, 30 (2): 26-29.银建中, 刘欣, 丁信伟. 超临界流体技术研究进展[J]. 江苏化工, 2002, 30 (2): 26-29.
[4] ZHU Z Q. Supercritical fluid technology—principal and application [M]. Beijing: Chemical Industry Press, 2000.朱自强. 超临界流体技术──原理和应用[M]. 北京: 化学工业出版社, 2000.
[5] SHEN Y, LEI W N, QIAN H F, et al. Research progress and coating preparation technology by supercritical fluid [J]. Machine Design and Manufacturing Engineering, 2015, 44 (1): 1-5.沈宇, 雷卫宁, 钱海峰, 等. 超临界流体镀层制备技术及其研究进展[J]. 机械设计与制造工程, 2015, 44 (1): 1-5.
[6] Supercritical fluid [EB/OL]. (2016–12–27) [2016–11–10]. http://www.baike.com/wiki/%E8%B6%85%E4%B8%B4%E7%95%8C%E6%B5%81%E4%BD%93 &prd=so_auto_doc_list.超临界流体[EB/OL]. (2016–12–27) [2016–11–10]. http://www.baike.com/wiki/%E8%B6%85%E4%B8%B4%E7%95%8C%E6%B5%81%E4%BD%93&prd= so_auto_doc_list.
[7] FANG J L. Supercritical fluid cleaning technology for PCB and electronic components [J]. Preinted Circuit Information, 2016, 24 (4): 52-56.方景礼. 印制板和电子元件的超临界流体清洗技术[J]. 印制电路信息, 2016, 24 (4): 52-56.
[8] GUO Y Q, SONG R G, CHEN L, et al. Effect of nanoscale alumina additive on microstructure as well as corrosion resistance and wear resistance of electroplated copper–tin alloy coatings [J]. Journal of Materials Protection, 2015, 48 (2): 1-4.郭燕清, 宋仁国, 陈亮, 等. 纳米Al2O3添加剂含量对Cu–Sn合金镀层微结构及性能的影响[J]. 材料保护, 2015, 48 (2): 1-4.
[9] ZHANG S Y. Wash parts of new industrial device with supercritical carbon dioxide [J]. Hebei Chemcal Engineering and Industry, 1994 (2): 12.张士莹. 用超临界CO2清洗部件的新型工业装置[J]. 河北化工, 1994 (2): 12.
[10] WANG Z J, SHEN Y L. Cleaning technique with supercritical carbon dioxide [J]. Cleaning World, 2005, 21 (11): 19-21.王仲军, 沈玉龙. 超临界CO2清洗技术[J]. 清洗世界, 2005, 21 (11): 19-21.
[11] LI Z Y, LIU X W, ZHANG X D, et al. Application of supercritical fluid in microelectronics cleaning [J]. Cleaning Technology, 2004, 2 (5): 5-10.李志义, 刘学武, 张晓冬, 等. 超临界流体在微电子器件清洗中的应用[J]. 洗净技术, 2004, 2 (5): 5-10.
[12] FANG J L. Theory & Application of Coordination Compounds in Elecrtoplating [M]. Beijing: Chemical Industry Press, 2008.方景礼. 电镀配合物──理论与应用[M]. 北京: 化学工业出版社, 2008.
[13] FANG J L. Theory & Application of Electroplating Additives [M]. Beijing: National Defence Industry Press, 2006.方景礼. 电镀添加剂理论与应用[M]. 北京: 国防工业出版社, 2006.
[14] LI Q S, WANG X W, YANG D Z, et al. Progress of manufacture of superfine powder by fast expending of supercritical liquid flow [J]. China Powder Science and Technology, 2006, 12 (2): 37-41.李青山, 王新伟, 杨德治, 等. 超临界流体制备超微粉体的研究进展[J]. 中国粉体技术, 2006, 12 (2): 37-41.
[15] ISHIZAKI T, MASUDA Y, SAKAMOTO M. Corrosion resistance and durability of superhydrophobic surface formed on magnesium alloy coated with nanostructured cerium oxide film and fluoroalkylsilane molecules in corrosive NaCl aqueous solution [J]. Langmuir, 2011, 27 (8): 4780-4788.
[16] ZONG Y F, WATKINS J J. Deposition of copper by the H2-assisted reduction of Cu(tmod)2in supercritical carbon dioxide: kinetics and reaction mechanism [J]. Chemistry of Materials, 2005, 17 (3): 560-565.
[17] BLACKBURN J M, LONG D P, CABAÑAS A, et al. Deposition of conformal copper and nickel films from supercritical carbon dioxide [J]. Science, 2001, 294 (5540): 141-145.
[18] ZHAO B, ZHAO M T, ZHANG Y F, et al. Deposition of Cu seed layer film by supercritical fluid deposition for advanced interconnects [J]. Chinese Physics B, 2013, 22 (6): 064217.
[19] WANG Y L, ZHANG Z W, LI B, et al. Preparation and structure characterization of Pd thin films by supercritiacal fluid deposition [J]. Acta Physica Sinica, 2011, 60 (8): 088103.王燕磊, 张占文, 李波, 等. 金属Pd薄膜的超界流体沉积制备及其结构表征[J]. 物理学报, 2011, 60 (8): 088103.
[20] SUN Z Y, LIU Z M, HAN B X, et al. Decoration carbon nanotubes with Pd and Ru nanocrystals via an inorganic reaction route in supercritical carbon dioxide–methanol solution [J]. Journal of Colloid and Interface Science, 2006, 304 (2): 323-328.
[21] WATANABE M, AKIMOTO T, KONDOH E. Synthesis of platinum–ruthenium alloy nanoparticles on carbon using supercritical fluid deposition [J]. Journal of Solid State Science and Technology, 2013, 2 (1): M9-M12.
[22] CHANG T-F M, NAGOSHI T, ISHIYAMA C, et al. Intact ultrathin Ni films fabricated by electroplating with supercritical CO2emulsion [J]. Applied Mechanics and Materials, 2013, 284/285/286/287: 147-151.
[23] KINASHI H, NAGOSHI T, CHANG T-F M, et al. Mechanical properties of Cu electroplated in Supercritical CO2emulsion evaluated by micro-compression test [J]. Microelectronic Engineering, 2014, 121: 83-86.
[24] CHANG T-F M, SONE M. Function and mechanism of supercritical carbon dioxide emulsified electrolyte in nickel electroplating reaction [J]. Surface and Coatings Technology, 2011, 205 (13/14): 3890-3899.
[25] LIU L Q, WANG C Y, JIANG B, et al. Preparation and properties of electroformed layer under supercritical state [C] // Chinese Mechanical Engineering Society. Proceedings of the 15th National Conference on Special Processing in China (last volume). [S.l.: s.n.], 2013: 41-43刘丽琴, 王创业, 姜博, 等. 超临界状态下复合电铸层制备及其性能研究[C] // 中国机械工程学会. 第15届全国特种加工学术会议论文集(下). [出版地不详: 出版者不详], 2013: 41-43.
[26] XIANG B T, WANG T, LIU J, et al. Treatment of sulphur-containing wastewater by supercritical water oxidation process [J]. Environmental Protection of Chemical Industry, 1999, 19 (2): 75-79.向波涛, 王涛, 刘军, 等. 超临界水氧化法处理含硫废水[J]. 化工环保, 1999, 19 (2): 75-79.
[27] WANG T, YANG M, XIANG B T, et al. Investigation of the elimination of organic substances in urine by supercritical water oxidation [J]. Space Medicine & Medical Engineering, 1997, 10 (5): 370-372.王涛, 杨明, 向波涛, 等. 超临界水氧化法去除尿素液中有机物的探索[J]. 航天医学与医学工程, 1997, 10 (5): 370-372.
[28] RICE S F, STEEPER R R. Oxidation rates of common organic compounds in supercritical water [J]. Journal of Hazardous Materials, 1998, 59 (2/3): 261-278.
[29] PÉREZ I V, ROGAK S, BRANION R. Supercritical water oxidation of phenol and 2,4-dinitrophenol [J]. The Journal of Supercritical Fluids, 2004, 30 (1): 71-87.
[30] AKI S, ABRAHAM M A. Catalytic supercritical water oxidation of pyridine: comparison of catalysts [J]. Industrial & Engineering Chemistry Research, 1999, 38 (2): 358-367.
[31] XU Y, GONG W J, JIANG P H, et al. Present research status of overseas surpercritical water oxidation [J]. Industrial Water & Wastewater, 2007, 38 (6): 8-11, 15.徐玥, 龚为进, 姜佩华, 等. 国外超临界水氧化技术的研究现状[J]. 工业用水与废水, 2007, 38 (6): 8-11, 15.
[32] SHANABLEH A. Supercritical water—a useful medium for waste destruction [J]. Arab Gulf Journal of Scientific Research, 1996, 14 (3): 543-556.
[33] GOTO M, NADA T, OGATA A, et al, Supercritical water oxidation for the destruction of municipal excess sludge and alcohol distillery wastewater of molasses [J]. The Journal of Supercritical Fluids, 1998, 13 (1/2/3): 277-282.
[34] JIANG T, ZHANG J, LI F Y. Environment-friendly new technology — supercritical water oxidation [J]. Pollution Control Technology, 2008, 21 (1): 69-71, 93.江涛, 张建, 李方圆. 环境友好型新技术──超临界水氧化法[J]. 污染防治技术, 2008, 21 (1): 69-71, 93.
[35] MATSUMURA Y, NUNOURA T, URASE T, et al. Supercritical water oxidation of high concentrations of phenol [J]. Journal of Hazardous Materials, 2000, 73 (3): 245-254.
[36] WANG J F, LI J, YANG J G. A new process for recovering copper from waste printed circuit board [J]. Hydrometallurgy of China, 2012, 31 (2): 106-109.王继峰, 李静, 杨建广. 一种从废旧电路板中回收铜的新工艺[J]. 湿法冶金, 2012, 31 (2): 106-109.
[37] BAI Q Z, WANG H, HAN J, et al. The status of technology and research of mechanical recycling of printed circuit board scrap [J]. Techniques and Equipment for Environmental Pollution Control, 2001, 2 (1): 84-89.白庆中, 王晖, 韩洁, 等. 世界废弃印刷电路板的机械处理技术现状[J]. 环境污染治理技术与设备, 2001, 2 (1): 84-89.
[38] GU X S. Copper Clad Laminates for Printed Circuit [M]. Beijing: Chemical Industry Press, 2002.辜信实. 印制电路用覆铜箔层压板[M]. 北京: 化学工业出版社, 2002.
[39] LIU Z F, HU Z X, LI H, et al. Research on recycling process and method of printed circuit board [J]. China Resources of Comprehensive Utilization, 2007, 25 (2): 17-21.刘志峰, 胡张喜, 李辉, 等. 印刷线路板回收工艺与方法研究[J]. 中国资源综合利用, 2007, 25 (2): 17-21.
[40] LIAO C H, ZHU L Y, FANG X, et al. Aapplications of supercritical water oxidation in treatment of dyeing waste water with high concentration and bad degradation [J]. Texitle Auxliaries, 2008, 25 (12): 22-26.廖传华, 诸旅云, 方向, 等. 超临界水氧化法在高浓度难降解印染废水治理中的应用[J]. 印染助剂, 2008, 25 (12): 22-26.
[41] ZHOU H Y, JIANG W L, WU H S, et al. Supercritical water oxidation for treatment of organic waste and trend analysis [J]. Environmental Science and Technology, 2012, 25 (6): 66-68.周海云, 姜伟立, 吴海锁, 等. 超临界水氧化有机废物研究应用现状及趋势分析[J]. 环境科技, 2012, 25 (6): 66-68.
[42] MA C Y, JIANG A X, PENG Y L, et al. Establishment and review of a pilot-plant-scale experimental set for supercritical water oxidation [J]. Chemical Industry and Engineering Progress, 2003, 22 (10): 1102-1104.马承愚, 姜安玺, 彭英利, 等. 超临界水氧化法中试装置的建立和考察[J]. 化工进展, 2003, 22 (10): 1102-1104.
[ 编辑:温靖邦 ]
声明
《电镀与涂饰》杂志创刊于1982年,由广州大学、广州市二轻工业科学技术研究所主办,是中文核心期刊、中国科技核心期刊、中国科学引文数据库来源期刊、中国期刊方阵双百期刊。《电镀与涂饰》杂志在工商部门的登记名称及收款账号为“广州镀涂文化传播有限公司”(营业执照编号:440108000033415)。
近期有读者(作者)向编辑部反映,通过“百度”等搜索引擎搜索到的杂志网站,要求预交几百或上千的文章发表费,并承诺保证可以发表,待收到费用之后,网站就失去联系。
在此,《电镀与涂饰》编辑部特别声明:作为核心期刊,《电镀与涂饰》杂志有严格的审稿及编辑流程,所有文章均需经过三审(初审-复审-终审),符合要求方能发表。《电镀与涂饰》编辑部从不收取所谓的“保证发表”费用。
友情提示:假冒网站的联系方式通常只留有电子邮箱或QQ等,不会留有联系电话。希望各位在付款之前,都能通过电话确认一下。
《电镀与涂饰》杂志唯一官方网站:www.plating.org。
联系电话:020–61302516 / 61302803。官方认证微信号:ddyts1982。
TQ153; TG178
:B
:1004 – 227X (2017) 04 – 0175 – 15
面向未来的表面精饰新技术──超临界流体技术
// 方景礼
评述了超临界流体技术的原理、特点以及在表面精饰领域的应用和进展,重点介绍了超临界CO2清洗硅晶圆,超临界萃取提纯电镀和化工中间体,超临界流体制备纳米微粒,超临界流体电沉积和化学沉积,超临界水氧化技术在处理各种难降解有机废水、工业污泥、废旧印刷电路板等方面的应用,以及超临界流体处理设备上的进展。事实说明,超临界流体技术在表面精饰行业有广阔的应用前景,是古老的表面精饰行业向技术更先进,工艺更环保,镀层质量更优异方向迈进的新途径。
超临界流体;表面精饰;清洗;电沉积;化学镀;复合镀;氧化;废水处理
南京大学,南京210023,中国

10.19289/j.1004-227x.2017.04.001
The Chinese version of this paper was published in the Vol.36 Issue No.1 ofElectroplating & Finishing.
Received data: 2016–11–10 Revised date: 2017–01–14
Biography: Prof. Fang Jing-li (1940–), male, graduated from chemistry department of Nanjing University in 1962, and got Master degree in 1965 from the same department under the tutelage of Professor Dai Anbang, one of the founder of Chinese Chemical Society and an academician of Chinese Academy of Sciences. He was working in chemistry department of Nanjing University from 1965 to 1995, as a principal engineer in Gul Technologies Singapore Ltd. and as a chief technology officer in Plaschem Pte. Ltd. (Singapore) in 1995-2002, as a senior technical consultant in Taiwan Uyemura Co., Ltd. in 2002-2004, as a technical director in Chartermate International Ltd. (Hong Kong) in 2005-2007, and as a Clean Production Specialist of Environment Science Society of Guangdong Province, a Clean Production Specialist of Environment Estate Society of Shenzhen, a Principal Specialist of Surface Engineering Society of Fujian Province and a senior technical consultant in Singapore Epson Ltd. after 2008. He was awarded the “Lifetime Achievement Award” in September 2016 in the 19th Interfinish World Congress & Exhibition (Interfinish 2016).
E-mail address: 13823673739@163.com。

