氟啶虫胺腈亚致死浓度对桃蚜生长和繁殖的影响
王泽华,范佳敏,陈金翠,宫亚军,魏书军
(北京市农林科学院植物保护环境保护研究所,北京 100097)
氟啶虫胺腈亚致死浓度对桃蚜生长和繁殖的影响
王泽华,范佳敏,陈金翠,宫亚军,魏书军
(北京市农林科学院植物保护环境保护研究所,北京 100097)
【目的】氟啶虫胺腈属第 4代新烟碱类药剂,研究旨在探讨氟啶虫胺腈亚致死浓度对 F0(当代)及F1(第1代)桃蚜(Myzus persicae)生长发育和繁殖的影响,为氟啶虫胺腈的合理使用提供理论依据。【方法】氟啶虫胺腈对桃蚜的亚致死剂量采用波特喷雾塔法确定,将琼脂铺于玻璃培养皿底部,再将新鲜油菜叶片背面向上铺在琼脂上。挑取15头成蚜至油菜叶片上,置于波特喷雾塔下喷雾,药剂设置7个浓度,处理48 h后检查桃蚜死亡情况,采用POLO-Plus10.0软件计算 LC10和LC25。采用建立生命表的方法评估氟啶虫胺腈亚致死剂量对桃蚜生长发育和繁殖的影响。评估药剂对F0代桃蚜的影响时,分别以LC10和LC25喷施成蚜,48 h后将成蚜移至未着药叶片,单头饲养直至死亡。评估药剂对F1代桃蚜的影响时,以LC10和LC25处理成蚜,48 h后将成蚜移至未着药叶片待其产蚜,24 h后随机选取初产若蚜单头饲养直至死亡,记录蚜虫存活及繁殖情况。采用SPSS 16.0软件分析若蚜发育历期、成蚜寿命、单雌产蚜量及生命表参数差异显著性。【结果】根据室内生物测定结果,氟啶虫胺腈对桃蚜成蚜48 h的LC10和LC25分别为0.012和0.041 mg·L-1。亚致死浓度的氟啶虫胺腈显著降低了F0和F1代桃蚜成蚜寿命、单雌产蚜量和产蚜历期,并表现为随药剂浓度增加,成蚜寿命、产蚜历期缩短,单雌产蚜量降低。LC10和LC25浓度处理F0代桃蚜后,成蚜寿命分别为20.89和15.47 d,均显著低于对照的25.41 d;单雌产蚜量分别为56.51和27.33头,显著低于对照的71.02头;产蚜历期分别为20.74和14.37 d,显著低于对照的25.27 d;F1代成蚜寿命分别为14.80和9.76 d,产蚜历期分别为12.03和8.59 d,单雌产蚜量分别为46.20和28.23头。与对照相比,LC10浓度的氟啶虫胺腈处理显著延长了F1代1龄若蚜的发育历期(1.73和2.21 d),LC25浓度的氟啶虫胺腈处理显著延长了2龄若蚜的发育历期(1.43和1.58 d),其余龄期及整个若蚜期发育历期与对照相比均无显著差异。生命表参数分析表明,氟啶虫胺腈 LC10和 LC25浓度处理后桃蚜净增殖率R0与对照相比显著降低,R0分别为 47.15、24.55和 64.47。【结论】亚致死剂量的氟啶虫胺腈能够显著抑制 F0及F1代桃蚜的寿命和繁殖力。
氟啶虫胺腈;新烟碱类杀虫剂;桃蚜;生命表;生物测定;波特喷雾塔
0 引言
【研究意义】桃蚜(Myzus persicae)是世界性重要经济作物害虫,也是世界范围内抗药性最为突出的害虫之一[1-2]。该虫寄主范围广,不仅直接取食造成危害,还可以分泌蜜露,导致煤污病,并能够传播多种植物病毒[3-4],给农业生产造成严重影响。一直以来对桃蚜的防治以化学农药为主,目前桃蚜对常用杀虫剂均产生了不同程度的抗药性[5-10],给该虫的防控带来了极大困难。氟啶虫胺腈(sulfoxaflor)是美国陶氏益农公司开发的砜亚胺类杀虫剂[11],属第 4代新烟碱类药剂,是防治蚜虫、介壳虫和粉虱等刺吸式口器害虫的全新杀虫剂产品[12],研究氟啶虫胺腈亚致死浓度对桃蚜生长发育与繁殖的影响,对氟啶虫胺腈的合理使用和桃蚜的防治具有重要意义。【前人研究进展】氟啶虫胺腈作用于烟碱型乙酰胆碱受体(nAChR)内独特的结合位点[13-15],被杀虫剂抗性行动委员会(IRAC)认定为全新Group 4C类杀虫剂中的唯一成员[13]。杀虫剂施于田间后,在环境中的毒力随着时间延长会逐渐递减到亚致死剂量,从而产生亚致死效应。亚致死效应能够对昆虫的生长发育、繁殖、生态行为和抗药性等产生不同程度的影响。一些药剂的亚致死剂量能刺激害虫生殖,从而导致害虫的再猖獗,另一些药剂则对害虫的生长发育表现为抑制效应,如延长害虫的发育历期或导致发育畸形,降低蛹重、化蛹率、羽化率及繁殖率等[16]。新烟碱类药剂是目前防治桃蚜的首选药剂,国内外关于该类杀虫剂的亚致死效应已有研究报道。亚致死浓度的吡虫啉、呋虫咹、噻虫嗪和烯啶虫胺能够抑制棉蚜成蚜的寿命和繁殖力,显著提高第 1代有翅蚜虫的比例[17-19];豌豆蚜(Acyrthosiphon pisum)在吡虫啉亚致死剂量下寿命显著缩短、繁殖率降低、发育历期延长[20];吡虫啉和噻虫嗪亚致死剂量处理下荻草谷网蚜(Sitobion miscanthi)种群的净生殖率、发育历期、内禀增长率与对照相比均降低[21],但也有研究发现亚致死剂量吡虫啉对桃蚜有刺激生殖作用[22]。【本研究切入点】由于氟啶虫胺腈在靶标受体上的结合位点不同,并具有抗单氧化酶代谢分解的能力,因此氟啶虫胺腈与其他新烟碱类杀虫剂不存在交互抗性[23-24],是抗性管理方面的一个优选药剂。明确药剂的亚致死效应是评价该药药效以及评估农药管理风险的关键。国内尚无氟啶虫胺腈亚致死剂量对桃蚜影响的相关报道,该药与其他新烟碱类杀虫剂对蚜虫生长发育和繁殖的亚致死效应有何异同尚待研究。【拟解决的关键问题】采用喷雾塔法测定氟啶虫胺腈对桃蚜的亚致死浓度,研究亚致死浓度氟啶虫胺腈对F0(当代)及F1(第1代)桃蚜生长发育及繁殖的影响,为桃蚜的综合防治及氟啶虫胺腈的合理应用提供理论依据。
1 材料与方法
试验于 2016年在北京市农林科学院植物保护环境保护研究所昆虫毒理学实验室内进行。
1.1 供试虫源及药剂
供试桃蚜为实验室敏感品系,2010年采自北京市农林科学院温室内,在实验室内不接触任何农药用油菜(Brassica campestris)继代饲养至今。饲养条件为温度(23±1)℃,相对湿度 50%—65%,光周期 16L﹕8D。97.0%氟啶虫胺腈原药由美国陶氏益农公司生产。
1.2 氟啶虫胺腈亚致死剂量的确定
采用波特(Potter)喷雾塔进行生物测定。首先用丙酮将97.0%氟啶虫胺腈原药溶解,然后用0.1% Trion X-100稀释成1 000 mg·L-1的母液,依次稀释成 0.025、0.05、0.1、0.2、0.4、0.8、1.6 mg·L-1药液待用。将1%的琼脂铺于直径6 cm的玻璃培养皿底部,再将新鲜健康的油菜叶片打成圆片,背面向上铺在琼脂上。用小毛笔挑取15头健壮成蚜至油菜叶片上,置于波特喷雾塔下喷雾,压力为63 kPa,施药量为2 mL。每个浓度设置6次重复。处理48 h后检查桃蚜死亡率,毛笔轻触虫体,不能行动者视为死亡。
1.3 氟啶虫胺腈亚致死剂量对桃蚜生物学特性的影响
1.3.1 对 F0代桃蚜的影响 采用建立生命表的方法评估氟啶虫胺腈亚致死剂量对桃蚜生长发育和繁殖的影响。将桃蚜单头饲养,待发育至成蚜的第 1天,以氟啶虫胺腈亚致死剂量LC10和LC25,分别用喷雾塔喷施桃蚜成蚜,每15个成蚜置于一个培养皿中,喷施压力为63 kPa,施药量为2 mL。48 h后将成蚜移至新鲜未着药叶片,单头饲养在单个培养皿中(直径6 cm),每天更换新鲜叶片至死亡,记录存活及繁殖情况,每天及时移去新产的若蚜。每个浓度处理50头,重复4次。对照组成蚜用喷雾塔喷施0.1% Triton X-100。
1.3.2 对 F1代桃蚜的影响 用氟啶虫胺腈亚致死剂量LC10和LC25处理成蚜,48 h后将成蚜转移至新鲜叶片上待其产蚜,24 h后随机取一只若蚜至培养皿中单独饲养,每天更换新鲜叶片,记录该代蚜虫的发育、存活及繁殖情况,直至死亡。每个浓度下观察50头,重复4次。对照组桃蚜成蚜用喷雾塔喷施0.1% Triton X-100。
1.4 数据分析
采用 POLO-Plus10.0软件计算 LC10、LC25和LC50。采用SPSS 16.0软件(Turkey’s HSD法)分析不同处理组若蚜发育历期、成蚜寿命、繁殖力及生命表参数差异显著性。净增殖率R0=∑ lxmx,平均世代历期T =∑ x lxmx/ R0,内禀增长率rm= (ln R0) / T,周限增长率λ= exp (rm),种群加倍时间t= ln 2/ rm,其中,x为时间间隔(d),lx表示个体在 x期间的存活率,mx表示个体在x期间的平均单雌产蚜量(只)[25]。
2 结果
2.1 氟啶虫胺腈对桃蚜的亚致死剂量
根据室内生物测定结果,计算得到氟啶虫胺腈对桃蚜成蚜48 h的LC10、LC25和LC50分别为0.012、0.041和0.157 mg·L-1(表1),其中LC10、LC25浓度后续试验处理。
2.2 氟啶虫胺腈亚致死剂量对 F0代桃蚜成蚜寿命和繁殖率的影响
各亚致死浓度处理组 F0代成蚜的寿命均显著低于对照组(F=144.13,P<0.05),单雌产蚜量均显著低于对照组(F=416.01,P<0.05),产蚜历期也均显著低于对照组(F=308.28,P<0.05)(表2)。LC25处理的成蚜在寿命、产蚜历期以及单雌产蚜量上也显著低于LC10处理组,表现为随药剂浓度增加,成蚜寿命、产蚜历期缩短,单雌产蚜量降低。亚致死浓度LC10和LC25处理的成蚜比对照组的存活率下降的稍显急剧(图 1)。以上结果表明亚致死浓度的氟啶虫胺腈能显著缩短桃蚜成蚜的寿命及产蚜历期,降低单雌产蚜量。

表1 氟啶虫胺腈对桃蚜成蚜48 h的亚致死剂量Table 1 Sublethal concentrations of sulfoxaflor to M. persicae adults after 48 h

表2 亚致死浓度氟啶虫胺腈对桃蚜生物学特性的影响Table 2 Effect of sublethal concentration of sulfoxaflor on biological traits of M. persicae adults
2.3 氟啶虫胺腈亚致死剂量对 F1代桃蚜生长发育的影响
亚致死浓度氟啶虫胺腈处理对桃蚜3龄、4龄历期均无显著影响。与对照相比,LC10浓度氟啶虫胺腈处理显著延长了1龄若蚜的发育历期(F=22.52,P<0.05),LC25浓度氟啶虫胺腈处理显著延长了 2龄若蚜的发育历期(F=46.20,P<0.05),其余与对照相比均无显著差异(表3)。亚致死浓度LC10和LC25处理的F1代桃蚜比对照组的存活率在第0—13天差异不明显,随后LC10和LC25处理组与对照相比表现出明显的急剧下降趋势(图 2)。以上结果表明氟啶虫胺腈亚致死剂量对若蚜的生长发育和存活率均没有显著影响,但对成蚜阶段存活率存在一定影响。

图1 亚致死浓度氟啶虫胺腈对F0代桃蚜逐日存活率的影响Fig. 1 Age-specific survival rate (lx) of sublethal concentrations of sulfoxaflor to F0M. persicae adults
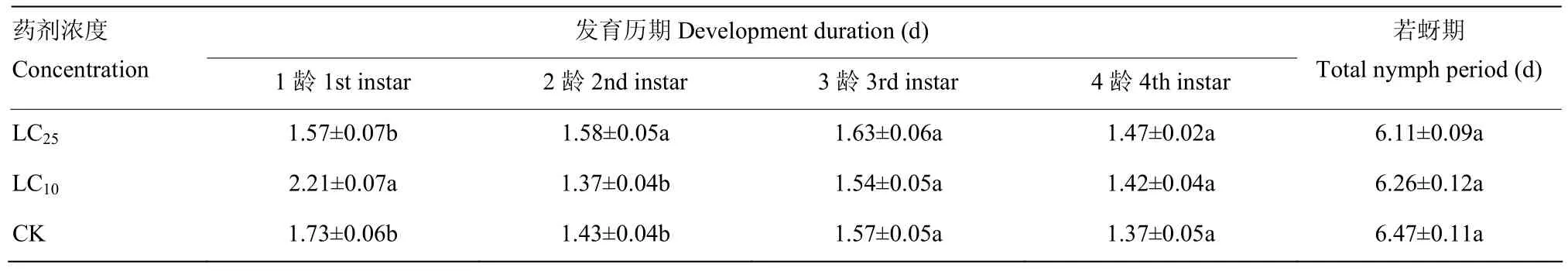
表3 亚致死浓度氟啶虫胺腈对桃蚜F1代若蚜生长发育的影响Table 3 Effect of sublethal concentrations of sulfoxaflor on nymph development of F1M. persicae

图2 亚致死浓度氟啶虫胺腈对F1代桃蚜逐日存活率的影响Fig. 2 Age-specific survival rate (lx) of sublethal concentrations of sulfoxaflor to F1M. persicae
2.4 氟啶虫胺腈亚致死剂量对 F1代桃蚜寿命及繁殖的影响
各亚致死浓度处理组成蚜的寿命均显著低于对照组(F=138.62,P<0.05),单雌产蚜量均显著低于对照组(F=210.53,P<0.05),产蚜历期也均显著低于对照组(F=109.84,P<0.05)。LC25处理的桃蚜成蚜在产蚜历期和单雌产蚜量上也显著低于LC10处理组,表现为随药剂浓度增加,产蚜历期缩短,单雌产蚜量降低(表 4)。以上结果表明亚致死浓度的氟啶虫胺腈能显著缩短桃蚜F1代成蚜的寿命及产蚜历期,降低其单雌产蚜量。
2.5 亚致死浓度氟啶虫胺腈对桃蚜种群生命表参数的影响
与对照相比,亚致死浓度LC25氟啶虫胺腈处理的净增殖率 R0显著降低(F=133.92,P<0.05),平均世代历期明显缩短(F=32.689,P<0.05),内禀增长率rm、周限增长率λ显著增高(F=7.758,P<0.05),种群加倍时间t减少。LC10处理组净增殖率R0与对照组相比显著降低(F=157.63,P<0.05),其他参数没有明显差异(表5)。
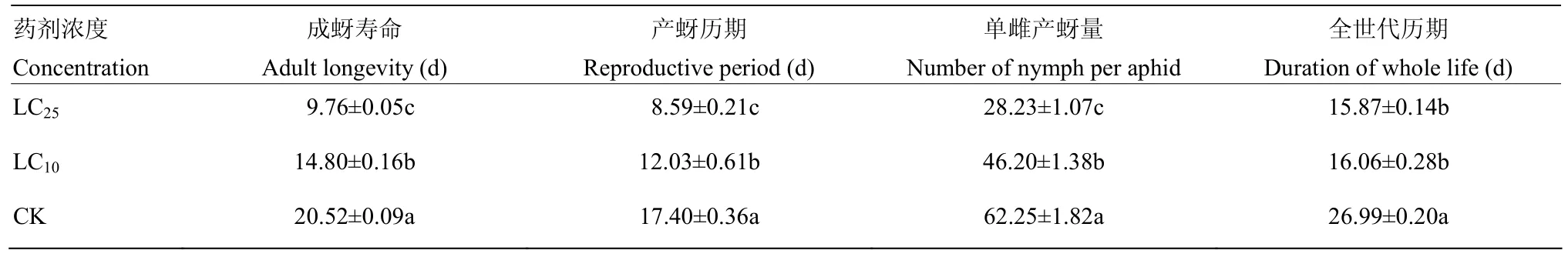
表4 亚致死浓度氟啶虫胺腈对F1代桃蚜成蚜生物学特性的影响Table 4 Effect of sublethal concentrations of sulfoxaflor on biological traits of F1M. persicae

表5 亚致死浓度氟啶虫胺腈处理后桃蚜的种群生命表参数Table 5 Life table parameters of M. persicae treated by sublethal concentrations of sulfoxaflor
3 讨论
氟啶虫胺腈属于第 4代新烟碱类杀虫剂,尤其对于已经对其他新烟碱类杀虫剂产生抗性的刺吸式害虫,具有良好的防效[13]。本研究表明,亚致死浓度的氟啶虫胺腈能够显著降低 F0和 F1代成蚜的寿命和繁殖力,但对F1代若蚜的生长发育没有显著影响。生命表参数分析表明,LC10和LC25处理组的净增殖率R0与对照相比均显著降低,同时存在浓度依赖性特点。LC25浓度处理后的平均世代历期T与对照相比显著缩短,内禀增长率rm增高,种群加倍时间t减少。
新烟碱类药剂是目前防治桃蚜的首选药剂,关于该类杀虫剂对蚜虫的亚致死效应研究已取得了一系列进展。JAM等[17]发现亚致死浓度的吡虫啉能够抑制棉蚜(Aphis gossypii)质量增加和蜜露分泌,并显著抑制棉蚜成蚜寿命、内禀增长率、净增殖率、平均世代和种群加倍时间。亚致死浓度的呋虫咹、噻虫嗪和烯啶虫胺均能够抑制棉蚜成蚜寿命和繁殖力[18];LASHKARI等[26]评估了亚致死浓度的吡虫啉和吡蚜酮对白菜蚜虫的影响,发现在单雌产蚜量和成蚜寿命上,处理组明显降低,内禀增长率降低,平均世代和种群加倍时间相比对照有所降低,而本研究中氟啶虫胺腈处理的桃蚜内禀增长率和周限增长率相比对照有所升高,这可能与该浓度处理下成蚜寿命明显缩短有关。WANG等[19]用LC25浓度的吡虫啉处理桃蚜后,发现F1代有翅蚜虫的比例显著提高;曾春祥等[27]报道了吡虫啉亚致死剂量能够抑制F0和F1代桃蚜种群的寿命和繁殖力;惠婧婧等[20]对吡虫啉对豌豆蚜的亚致死效应研究发现,随着吡虫啉亚致死剂量的增加,F0代及F1代豌豆蚜成蚜寿命显著缩短,产蚜量显著降低,发育历期、平均世代周期和种群加倍时间延长。吡虫啉和噻虫嗪亚致死剂量处理下荻草谷网蚜种群的净生殖率、发育历期、内禀增长率和周限增长率与对照相比均降低,种群加倍时间明显延长[21]。啶虫脒、呋虫胺、吡虫啉、烯啶虫胺和噻虫嗪均属于烟碱乙酰胆碱受体激动剂中的Group 4A类杀虫剂,以上新烟碱类杀虫剂亚致死剂量对不同种类蚜虫均能够抑制成蚜寿命及产蚜量,但对于种群生命表参数间存在不同影响。氟啶虫胺腈属Group 4C类杀虫剂,TANG等[28]也评估了亚致死浓度(LC25)的氟啶虫胺腈对桃蚜的影响,该研究中采用的亚致死浓度(LC25)为0.009 mg·L-1,与本研究中的LC10(0.012 mg·L-1)数值相近,该研究发现处理组成蚜与对照相比在寿命和繁殖力方面没有显著性差异,F1代桃蚜各龄期发育历期、成蚜寿命和繁殖力方面与对照相比亦没有显著差异,但成蚜前期和产卵前期的时间与对照相比显著延长,这可能和试虫种群不同有关。
本研究表明亚致死浓度的氟啶虫胺腈不但对 F0代桃蚜有显著影响,而且对F1代桃蚜同样具有显著抑制作用,这可能与蚜虫的生殖方式有一定的关系。蚜虫是一种重代生殖的昆虫,也就是说,孤雌生殖的母体内的若蚜体内也孕育着自己的F1代,具有“三代一体”的特征[29],因此对桃蚜 F0的处理可能造成多代(F0、F1、F2)的影响。
4 结论
亚致死浓度的氟啶虫胺腈能够显著降低 F0和 F1代桃蚜成蚜的寿命、单雌产蚜量和产蚜历期,并表现为随药剂浓度增加,成蚜寿命、产蚜历期缩短,单雌产蚜量降低。LC10浓度的氟啶虫胺腈处理显著延长了F1代1龄若蚜的发育历期,LC25浓度的氟啶虫胺腈处理显著延长了2龄若蚜的发育历期。生命表参数分析表明,LC10和LC25处理组的净增殖率R0与对照相比均显著降低。
[1] EMDEN H, EASTOP V, HUGHES R, WAY M. The ecology of Myzus persicae. Annual Review of Entomology, 1969, 14(1): 197-270.
[2] GARZO E, MORENO A, HERNANDO S, MARINO V, TORNE M, SANTAMARIA E, DIAZ I, FERERES A. Electrical penetration graph technique as a tool to monitor the early stages of aphid resistance to insecticides. Pest Management Science, 2016, 72(4): 707-718.
[3] NOVY R G, NASRUDDIN A, RAGSDALE D W, RADCLIFFE E B. Genetic resistances to Potato leafroll virus, Potato Virus Y, and green peach aphid in progeny of Solanum etuberosum. American Journal of Potato Research, 2002, 79(1): 9-18.
[4] LOWERY D T, VICKERS P M, BITTNER L A, STOBBS L W, FOOTTIT R G. Aphid transmission of the Ontario isolate of Plum pox virus. Journal of Economic Entomology, 2015, 108(5): 2168-2173.
[5] DEVONSHIRE A L, FIELD L M, FOSTER S P, MOORES G D,WILLIAMSON M S, BLACKMAN R. The evolution of insecticide resistance in the peach-potato aphid, Myzus persicae. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 1998, 353(1376): 1677-1684.
[6] 顾春波, 王刚, 王开运, 马惠, 郭庆龙. 我国西南烟区桃蚜Myzus persicae (Sulzer)的抗药性水平. 植物保护学报, 2006, 33(1): 77-80.
GU C B, WANG G, WANG K Y, MA H, GUO Q L. Studies on the resistance level of Myzus persicae (Sulzer) in main tobacco regions of southwestern China. Acta Phytophylacica Sinica, 2006, 33(1): 77-80. (in Chinese)
[7] 宫亚军, 王泽华, 石宝才, 康总江, 朱亮, 郭晓军, 刘建华, 魏书军.北京地区不同桃蚜种群的抗药性研究. 中国农业科学, 2011, 44(21): 4385-4394.
GONG Y J, WANG Z H, SHI B C, KANG Z J, ZHU L, GUO X J, LIU J H, WEI S J. Resistance status of Myzus persicae (Sulzer) (Hemiptera: Aphididae) populations to pesticide in Beijing. Scientia Agricultura Sinica, 2011, 44(21): 4385-4394. (in Chinese)
[8] FENG R, ISMAN M B. Selection for resistance to azadirachtin in the green peach aphid, Myzus persicae. Cellular & Molecular Life Sciences, 1995, 51(8): 831-833.
[9] LI Y, XU Z, SHI L, SHEN G, HE L. Insecticide resistance monitoring and metabolic mechanism study of the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae), in Chongqing, China. Pesticide Biochemistry & Physiology, 2016, 132: 21-28.
[10] SRIGIRIRAJU L, SEMTNER P J, ANDERSON T D, BLOOMQUIST J R. Esterase-based resistance in the tobacco-adapted form of the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae) in the eastern United States. Archives of Insect Biochemistry and Physiology, 2009, 72(2): 105-123.
[11] ZHU Y, LOSO M R, WATSON G B, SPARKS T C, ROGERS R B, HUANG J X, GERWICK B C, BABCOCK J M, KELLEY D, HEGDE V B. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. Journal of Agricultural & Food Chemistry, 2011, 59(7): 2950-2957.
[12] BABCOCK J M, GERWICK C B, HUANG J X, LOSO M R, NAKAMURA G, NOLTING S P, ROGERS R B, SPARKS T C, THOMAS J, WATSON G B. Biological characterization of sulfoxaflor, a novel insecticide. Pest Management Science, 2011, 67(3): 328-334.
[13] WATSON G B, LOSO M R, BABCOCK J M, HASLER J M, LETHERER T J, YOUNG C D, ZHU Y, CASIDA J E, SPARKS T C. Novel nicotinic action of the sulfoximine insecticide sulfoxaflor. Insect Biochemistry & Molecular Biology, 2011, 41(7): 432-439.
[14] SPARKS T C, WATSON G B, LOSO M R, GENG C, BABCOCK J M, THOMAS J D. Sulfoxaflor and the sulfoximine insecticides: chemistry, mode of action and basis for efficacy on resistant insects. Pesticide Biochemistry & Physiology, 2013, 107(1): 1-7.
[15] WANG N X, WATSON G B, LOSO M R, SPARKS T C. Molecular modeling of sulfoxaflor and neonicotinoid binding in insect nicotinic acetylcholine receptors: impact of the Myzus β1 R81T mutation. Pest Management Science, 2016, 72(8): 1467-1474.
[16] 全林发, 张怀江, 孙丽娜, 李艳艳, 闫文涛, 岳强, 仇贵生. 杀虫剂对害虫的亚致死效应研究进展. 农学学报, 2016, 6(5): 33-38.
QUAN L F, ZHANG H J, SUN L N, LI Y Y, YAN W T, YUE Q, QIU G S. Research advances in sublethal effect of pesticide. Journal of Agriculture, 2016, 6(5): 33-38. (in Chinese)
[17] JAM N A, KOCHEYLI F, MOSSADEGH M S, RASEKH A, SABER M. Lethal and sublethal effects of imidacloprid and pirimicarb on the melon aphid, Aphis gossypii Glover (Hemiptera: Aphididae) under laboratory conditions. Journal of Crop Production, 2014, 3(1): 89-98.
[18] SHI X, JIANG L, WANG H, QIAO K, WANG D, WANG K. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid, Aphis gossypii. Pest Management Science, 2011, 67(12): 1528-1533.
[19] WANG X Y, YANG Z Q, SHEN Z R, LU J, XU W B. Sublethal effects of selected insecticides on fecundity and wing dimorphism of green peach aphid (Hom., Aphididae). Journal of Applied Entomology, 2008, 132(2): 135-142.
[20] 惠婧婧, 刘长仲, 孟银凤, 陈洁. 吡虫啉对豌豆蚜的亚致死效应.植物保护, 2009, 35(5): 86-88.
HUI J J, LIU C Z, MENG Y F, CHEN J. Sublethal effects of imidacloprid to Acyrthosiphon pisum. Plant Protection, 2009, 35(5): 86-88. (in Chinese)
[21] 都振宝. 吡虫啉和噻虫嗪亚致死剂量对荻草谷网蚜生命表参数及取食行为的影响[D]. 武汉: 华中农业大学, 2012.
DU Z B. Effect of sublethal concentration of imidacloprid and thiamethoxam on the life-table parameters and feeding behavior of Sitobion miscanthi[D]. Wuhan: Huazhong Agricultural University, 2012. (in Chinese)
[22] CHRISTOPHER C G, RAMANAIDU K, ASTATKIE T, ISMAN M B. Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin. Pest Management Science, 2009, 65(2): 205-209.
[23] LONGHURST C, BABCOCK J M, DENHOLM I, GORMAN K, THOMAS J D, SPARKS T C. Cross-resistance relationships of the sulfoximine insecticide sulfoxaflor with neonicotinoids and other insecticides in the whiteflies Bemisia tabaci and trialeurodes vaporariorum. Pest Management Science, 2013, 69(7): 809-813.
[24] HERRON G A, LANGFIELD B J, BOGEMA D R, CHEN Y. Baseline susceptibility and cross-resistance in Aphis gossypii Glover (Aphididae: Hemiptera) to phorate and sulfoxaflor. Austral Entomology, 2014, 53(1): 32-35.
[25] 徐汝梅. 昆虫种群生态学. 北京: 北京师范大学出版社, 2007.
XU R M. Insect Population Ecology. Beijing: Beijing Normal University Press, 2007. (in Chinese)
[26] LASHKARI M R, SAHRAGARD A, GHADAMYARI M. Sublethal effects of imidacloprid and pymetrozine on population growth parameters of cabbage aphid, Brevicoryne brassicae on rapeseed, Brassica napus L. Insect Science, 2007, 14(3): 207-212.
[27] 曾春祥, 王进军, 曾智平, 曹高. 吡虫啉亚致死剂量对桃蚜实验种群的胁迫效应. 中国农学通报, 2006, 22(12): 335-338.
ZENG C X, WANG J J, ZENG Z P, CAO G. Impact of sublethal doses of imidacloprid on experimental population of Myzus persicae. Chinese Agricultural Science Bulletin, 2006, 22(12): 335-338. (in Chinese)
[28] TANG Q, XIANG M, HU H, AN C, GAO X. Evaluation of sublethal effects of sulfoxaflor on the green peach aphid (Hemiptera: Aphididae) using life table parameters. Journal of Economic Entomology, 2015, 108(6): 2720-2728.
[29] 赵惠燕, 汪世泽. 孤雌胎生棉蚜胚胎学观察. 昆虫知识, 1992, 29(1): 19-21.
ZHAO H Y, WANG S Z. Virginopara embryological observation of cotton aphid. Entomological Knowledge, 1992, 29(1): 19-21. (in Chinese)
(责任编辑 岳梅)
Sublethal Effects of Sulfoxaflor on the Growth and Reproduction of the Green Peach Aphid Myzus persicae
WANG ZeHua, FAN JiaMin, CHEN JinCui, GONG YaJun, WEI ShuJun
(Institute of Plant and Environmental Protection, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097)
【Objective】 Sulfoxaflor is the fourth generation of neonicotinoids. In this study, the sublethal effects of this pesticide on the development and reproduction of the F0(parental) and the F1(first generation) green peach aphid Myzus persicae were investigated to provide a theoretical basis for proper usage of this insecticide. 【Method】 The sublethal concentrations of LC10and LC25were determined by the bioassay method of Potter spray tower. The agar was placed at the bottom of the glass dish, whileleaf discs were placed with their adaxial surface downward onto agar. Fifteen adult aphids were transferred onto each leaf disc. Insecticide were sprayed to the leaf disc with aphids by using the Potter spray tower under seven concentrations. Mortality of the aphids was recorded 48 h later. LC10and LC25were estimated using POLO-Plus10.0 software. Sublethal effects of sulfoxaflor on the development and reproduction of the green peach aphid was evaluated by the method of establishing a life table. For the F0aphid, sulfoxaflor was sprayed on the adult aphids at the concentrations of LC10and LC25. After the application of the insecticide for 48 h, the adults were moved to fresh leaves without insecticide and reared separately until death. For the F1aphid, sulfoxaflor was sprayed on the adult aphids at the concentrations of LC10and LC25. After the application of insecticide for 48 h, the adults were moved to fresh leaves without insecticide. When the adult aphid produced nymphs for 24 h, one nymph was randomly selected and reared separately until death. The survival and reproduction of each aphid were recorded. The statistical differences of the development duration of nymphs, the adult longevity, the number of nymph per aphid and the life table parameters of F0and F1were analyzed using SPSS 16.0.【Result】 According to the bioassay, the LC10and LC25of sulfoxaflor on the green peach aphid after 48 h were 0.012 and 0.041 mg·L-1. Treatments with sublethal concentrations of sulfoxaflor significantly reduced the adult longevity, the number of nymph per aphid and the reproductive period of F0and F1. The values reduced with the increase of the concentration of insecticide. After being exposed to the sublethal concentrations LC10and LC25of sulfoxaflor, for the F0aphid, the average longevity of aphid adult was 20.89 and 15.47 d, respectively, shorter than that of control (25.41 d). The nymph number per aphid after treatment with LC10and LC25of sulfoxaflor was 56.51 and 27.33, respectively, significantly less than that of control (71.02), while the reproductive period was 20.74 and 14.37 d, respectively, significantly shorter than that of control (25.27 d). For the F1aphid, the average longevity of adult was 14.80 and 9.76 d, the reproductive period was 12.03 and 8.59 d, the nymph number per aphid after treatment with sulfoxaflor at LC10and LC25was 46.20 and 28.23, respectively. Compared with the control, treatment with sulfoxaflor at LC10significantly extended the development duration of 1st instar nymph (1.73 and 2.21 d), while treatment with sulfoxaflor at LC25significantly extended the development duration of 2nd instar nymph (1.43 and 1.58 d). However, there was no significantly difference in the other instar development duration and the total nymph period. Life table analysis showed that the net reproductive rate R0was decreased significantly after treatment with sulfoxaflor at LC10and LC25with the values of 47.15, 24.55, respectively, compared with the control with a value of 64.47. 【Conclusion】Sublethal concentrations of sulfoxaflor have inhibitory effects on adult longevity and fecundity of the F0and F1M. persicae.
sulfoxaflor; neonicotinoid; Myzus persicae; life table; bioassay; Potter spray tower
2016-09-18;接受日期:2016-11-28
北京市科委重大项目(D16110500550000)、北京市农林科学院科技创新团队(JNKYT201605)、北京市农林科学院创新能力建设专项(KJCX20150406)
联系方式:王泽华,E-mail:wangzehua200707@163.com。通信作者魏书军,E-mail:shujun268@163.com。通信作者宫亚军,E-mail:gongyajun200303 @163.com

