POTENTIAL RISKS OF HIGH LEVEL REPLACEMENT OF DIETARY FISH MEAL BY CANOLA MEAL ON LARGE YELLOW CROAKER LARIMICHTHYS CROCEA (RICHARDSON, 1846): GROWTH, HEALTH AND NUTRITIONAL VALUES AS A FOOD FISH
MENG Yu-Qiong, MIAO Xin, SUN Rui-Jian, MA Rui, SHENTU Ji-Kang, ZHANG Wen-Bingand MAI Kang-Sen
(1. The Key Laboratory of Aquaculture Nutrition and Feed, Ministry of Agriculture; the Key Laboratory of Mariculture (Ministry of Education); Fisheries College, Ocean University of China, Qingdao 266003, China; 2. Technology Center of Tongwei Co. LTD, Chengdu 610041, China; 3. College of Eco-Environmental Engineering, Qinghai University, Xining 810016, China; 4. Ningbo Ocean and Fisheries Research Institute, Ningbo 315010, China)
POTENTIAL RISKS OF HIGH LEVEL REPLACEMENT OF DIETARY FISH MEAL BY CANOLA MEAL ON LARGE YELLOW CROAKER LARIMICHTHYS CROCEA (RICHARDSON, 1846): GROWTH, HEALTH AND NUTRITIONAL VALUES AS A FOOD FISH
MENG Yu-Qiong1, MIAO Xin1, SUN Rui-Jian2, MA Rui3, SHENTU Ji-Kang4, ZHANG Wen-Bing1and MAI Kang-Sen1
(1. The Key Laboratory of Aquaculture Nutrition and Feed, Ministry of Agriculture; the Key Laboratory of Mariculture (Ministry of Education); Fisheries College, Ocean University of China, Qingdao 266003, China; 2. Technology Center of Tongwei Co. LTD, Chengdu 610041, China; 3. College of Eco-Environmental Engineering, Qinghai University, Xining 810016, China; 4. Ningbo Ocean and Fisheries Research Institute, Ningbo 315010, China)
A growth trial was conducted to evaluate the potential risks of high levels replacement of dietary fish meal by canola meal on large yellow croaker Larimichthys crocea (Richardson, 1846). It focused on the growth, health and nutritional values as a food fish. Diet with 60% of fish meal was formulated as the control group (FM). The other four experimental diets were formulated with graded replacement levels of fish meal by canola meal according to mass fraction. These replacement levels included 15% (CM15), 30% (CM30), 60% (CM60) and 100% (CM100), respectively. The 5 experimental diets were fed to 5 groups of large yellow croaker [initial weight of (135.38±1.02) g] for 12 weeks. There were 5 replicates per group. Results showed that the growth and feed conversion ratio (FCR) of large yellow croaker was not significantly influenced when the replacement level of dietary fish meal by canola meal were 15% and 30%. However, values of final body weight and the specific growth rate significantly decreased and value of FCR significantly increased in groups of CM60 and CM100 (P<0.05). Feed intake increased significantly, while condition factor significantly decreased in CM100 group (P<0.05). Histologically, less twist and disorganized morphology in intestine, distinct circular vacuoles and nuclear perturbations in liver were found when fish was fed with canola meal included diets. Through the digital X-ray analysis of skeleton, both vertebral body and mouth deformities were found in fish fed with canola meal. As for nutritional values, no significant difference was observed in lipid content, protein content and amino acid profile of dorsal muscle. While fatty acid compositions were significantly changed in muscle. N-6 fatty acid contents significantly increased, and DHA and EPA contents decreased (P<0.05). Based on the standards of the European Food Safety Authority (EFSA), however, these changes didn't influence the fact that large yellow croaker was also recommended as a healthy food fish. In summary, the negative impacts of high levels (60% and 100%) replacement of dietary fish meal by canola meal on large yellow croaker mainly related to the reduced growth performances, altered intestinal and hepatic morphology as well as impaired skeletal health. However, large yellow croaker fed with canola meal in diets still meet the standard of healthy food, thus canola meal replacement didn't significantly influence the nutritional value of large yellow croaker as a food fish.
Large yellow croaker; Canola meal; Fish meal; Growth; Health; Nutritional value
Continued growth of aquaculture production depends on the development of sustainable protein sources to replace fish meal in aqua feeds. As the second rank in global protein products for oils and meals, rapeseed/canola meal attracts broad attentions not only because of its low cost even compared with soybean meal, but also due to its well-balanced amino acids composition[1]. Unfortunately, certain limitations including presence of anti-nutritional factors are considered one of the main reasons limiting its further inclusion in diets. By improving the quality of rapeseed meal, canola meal becomes an outstanding candidate for fish meal replacement. Canola is the name given to genetically selected varieties of rapeseed (Brassica napus and Brassica campestris) that are low in both glucosinolates and erucic acid[2]. Compared with fish meal, the price of canola meal is much lower. And canola meal is also available worldwide[3]. Canola meal contains high (35—45 g/100 g) crude protein and has been one of the best amino acids balances of commercially available plant proteins with highest protein efficiency ratio of all plant based proteins[4,5]. Furthermore, canola meal is well known for containing relatively high levels of sulphur containing amino acids (e.g., Met and Cys), minerals (e.g., potassium, sulphur, calcium, iron, selenium and phosphorus) and vitamins (choline, biotin, folic acid, niacin, riboflavin and thiamin)[6]. However, some heat labile (e.g., glucosinolates) and heat stable (e.g., phytic acid, phenolic compounds and tannins) anti-nutritional factors as well as relatively high level of fiber content (about 12%) reduce its nutritional value[7].
Studies on nutritional evaluation of canola meal as an alternative to fish meal and its effects on the nutrient digestibility, growth performance and physiological responses of carnivorous fish (e.g., rainbow trout, Japanese sea bass and Chinook salmon) were well documented[8—10]. There are two ways to evaluate protein sources. Firstly, plant proteins were used to replace equal content of protein in fish meal. Due to their difference in protein and lipid level, other protein and lipid sources were supplemented to formulate isonitrogenous and isolipidic diets. However, except for amino acids and lipid content, knowledge on the effective pro-nutritional factors difference between fish meal and plant protein sources are still limited. Most of them mainly lie in taurine, cholesterol, nucleotides, essential fatty acids as well as some other uncharacterized factors associated with low molecular weight fractions[4]. Furthermore, the effects of anti-nutrients in plant protein sources are still not totally clear. Thus, the understanding of the effects of plant protein sources on fish was still limited. Secondly, fish meal was replaced by plant protein without balancing the protein, amino acid, lipid and fatty acid compositions in diet. It is helpful in comprehensively understanding the potential risks caused by a specific plant protein. In a study of assessing the safety of plant protein sources, Cai et al.[11]found that the high replacement level of dietary fish meal by rapeseed meal or peanut meal in a non-isonitrogenous and non-isoenergetic design not only retarded the growth but also produced health risks to crucian carp. To our knowledge, there was limited study to assessment the risks of total replacement of fish meal by equal amount of single plant protein sources regardless of the changes of protein and lipid contents in diet.
Large yellow croaker Larimichthys croceais was favored by a lot of people in China due to its goldenyellow skin, red lips and yellow fins. The farming of large yellow croaker has been under rapid development since the 1990s, and is now the first most popular mariculture fish species in China, with more than 127917 metric tons produced in 2014[12]. After about 13 months' rearing cycle, farmed large yellow croaker reaches its market size about 250 g[13]. The aim of the present study was to investigate the potential risks of extreme replacement of dietary fish meal by canola meal by dry matter weight basis on the growth performance, health and nutritional value of large yellow croaker. Considering the fact that higher plant protein tolerance ability of larger fish, large yellow croaker with initial body weight of about 135g was used in the present study. Furthermore, to avoid other feed stuffs than canola meal were supplemented into fish meal replacement diet, isonitrogenous and isoenergetic diets were not considered.
1 Materials and methods
1.1 Experimental diets
Diet formulation and compositions are shown in Tab. 1. Fish meal and canola meal were used as the main dietary protein sources. Control diet (60% of fish meal) without canola meal was named as FM. Based on the control diet, 90, 180, 360 and 600 g/kg of fish meal were replaced by equal levels of canola meal, respectively[11]. According to the percentage of replacement in the control fish meal content, the fish meal replacement levels are: 15%, 30%, 60% and 100%, which were named as CM15, CM30, CM60 and CM100, respectively.
1.2 Fish and feeding
The feeding trial was conducted in Sandu bay, Ningde, Fujian, China, in 2013. Large yellow croakerjuveniles were obtained from a local commercial hatchery. Prior to the feeding trial, fish were acclimatized in a floating sea cage (4.0 m×8.0 m×4.0 m) and fed with FM for two weeks. Then all the fish were starved for 24h. After anesthetized and weighed, groups of fish with similar size [initial body weight:(135.38±1.02) g] were randomly assigned to 25 cages (2 m×2 m×2.5 m). Each cage stocked with 65 fish was used as a replicate, and each diet had 5 replicates. Fish were hand-fed to apparent satiation twice daily at 05: 00 and 17: 00, respectively, for 12 weeks. During the feeding trial, feed consumption as well as the number and weight of dead fish were recorded daily. The water temperature was 27.5—32℃, salinity from 27‰ to 29‰, and dissolved oxygen was approximately 7 mg/L.
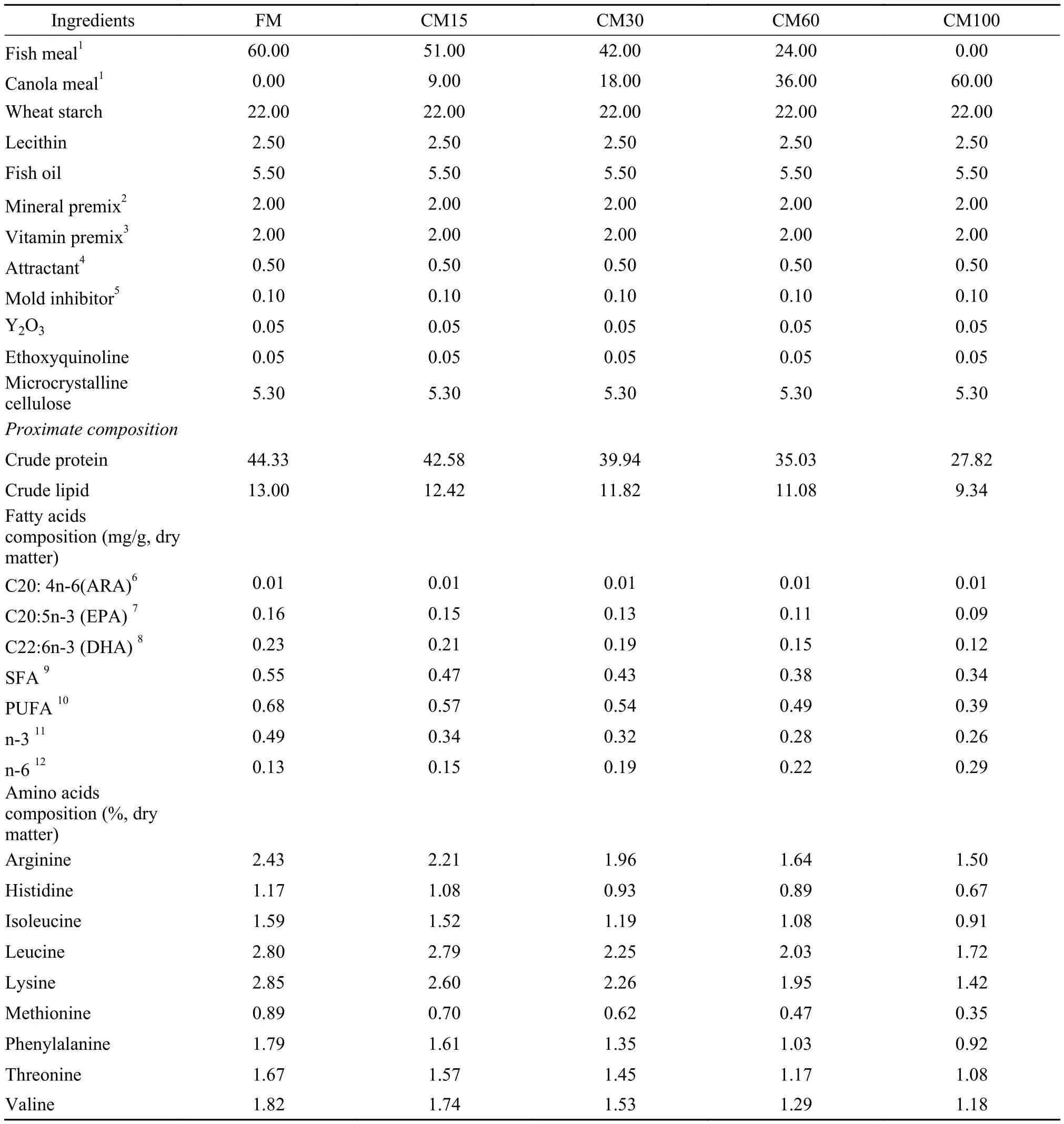
Tab. 1 Formulation and compositions of the experimental diets (% dry matter)
1.3 Sample collection
After the feeding trial, fish were fasted for 24h, and then were counted and weighed. Fifteen fish per treatment were randomly selected and stored at -20℃for the determination of the skeleton deformity. Eight fsh per cage were randomly sampled to detect body length and body weight to calculate the condition factor. After that, blood was drawn from the caudal vein of 3 fish per cage by using a 2 mL syringe, and allowed to clot for 5h at 4℃. The clot was removed and residual blood cells were separated from the straw-colored serum by centrifugation (3000×g for 15min at 4℃). Then the serum samples were stored at -80℃ for subsequent analysis. In the end, liver and viscera were rapidly excised and weighted. Another 3 fish per cage were randomly chosen and immediately put into the ice box, and then delivered to indoor laboratory. Dorsal muscle samples were taken and stored at -80℃ to determine the contents of protein and lipid as well as the compositions of amino acids and fatty acids. Two fish per cage were used for the histological analysis of liver and mid intestine.
1.4 Chemical analysis
Feed ingredients and experimental diets were analyzed for crude protein by using the standard methods of AOAC[14]. Crude protein was calculated from the determination of total nitrogen (N×6.25) by using the Kjeldahl method (2300-Auto-analyzer, FOSS, Denmark). Crude lipid was determined gravimetrically following ether extraction of the lipids according to Soxhlet method (36680-analyer, BUCHI, Switzerland).
To avoid the high muscle heterogeneity, about 10 g wet dorsal muscle per fish was used to determine the contents of crude protein[14]and lipid[15]as well as the compositions of amino acids and fatty acids. For determination of amino acids and fatty acids composition, experiment diets and muscle samples were dried to a constant weight at a lyophilizer (ALPHA 1—4 LD lyophilizer, Kleist, Germany). The amino acids composition were determined by automatic amino acids analyzer (Biochrom 30 Ltd, Cambridge, UK) after acid hydrolysis in 6 N HCl for 24h at 110℃. Based on the total lipid extraction previously, fatty acids composition were determined using lipids extraction by the method of Taha et al.[16]with some modifications. Briefly, fatty acids separation and identification was carried out on gas chromatography and mass spectrogram (GC-MS-QP2010, Shimadzu, Japan) fitted with an automatic sampler. The conditions were as follows: Rxi-1MS capillary column (30 m×0.25 mm, 0.25 μm); temperature programmed from 150℃ to 200℃ at 15℃/min, then from 200℃ to 250℃ at 2℃/min; injector at 250℃. Sample of 1 μL was injected in a split mode (20:1). Carrier gas was helium at 1.0 mL/min. Solvent cut time was 2.5min. Mass spectra were obtained under EI condition at 70 eV in the 45—500 m/z range. The ion source was held at 230℃ and interface at 280℃. Identification of fatty acids was based on mass spectra from library databases (NIST08.LIB), considering similar degree higher than 80%. Fatty acids were quantified by internal standard concentration and peak area ratio. The concentrations of fatty acids were expressed in milligram equivalents of internal standard for each gram of fish muscle (wet basis).
1.5 Liver and mid-intestine histology analysis
For histology analysis, liver samples were collected and placed into Bouin fxative solution for fixation. Then the samples were transferred into a 70% ethanol solution after 24h. The tissue samples were processed onto paraffin wax blocks and cut into 6 μm thick cross sections using a microtome and stained with hematoxylin and eosin for light microscopy examination (Olympus BX51; Tokyo Japan).
Mid intestine from FM, CM60 and CM100 group (two fish per cage, three cages per group) were used for examination by scanning electron microscopy (SEM). Briefly, fish intestine was open longitudinally and flushed three times by syringe with phosphate buffers (0.1 mol/L pH 7.4). After being fixed in 2.5% glutaraldehyde (phosphate buffered), samples were kept in refrigerator at 4℃. Prior to post fixation, samples were washed with phosphate buffer and then fixed in 1% osmic acid (phosphate buffered) for 90min. After being washed three times in phosphate buffer, the samples were dehydrated in graded ethanol and dried with critical point dryer (XD-1, Eiko, USA) and sputter-coated with gold (IB-3, Eiko, USA). These specimens were then observed by SEM (JSM-840, JEOL, USA).
1.6 Skeleton morphology analysis
Fish were put in the ice boxes overnight to totally thawing, and then digital X-ray using standard settings (Uni-Vision. Shimadzu, Japan) were used to determine skeleton deformities. The characterizations ofthe different deformities were performed on the basis of the previous studies[17,18].
1.7 Calculations
Feed intake (FI, %/d)=100×feed fed/[days of feeding trial×(final body weight+initial body weight)/2]
Feed conversion ratio (FCR)=feed intake/weight gain
Specific growth rate (SGR, %/d)=100×ln (final body weight/initial body weight)/days of feeding trial
Survival rate (SR, %)=(final amount of fish/initial amount of fish)×100
Hepato-somatic index (HSI, %)=(Hepatic weight/final body weight)×100
Viscero-somatic index (VSI, %)=(viscera weight/final body weight)×100
Condition factor (CF, %)=(final body weight/ body length3)×100
1.8 Statistical analysis
Data were presented as means±S.E.M. (standard error of the mean). One-way ANOVA was conducted for all the data using SPSS 17.0 for windows. When overall differences were significant (P<0.05), Tukey's test was conducted to compare the means between individual treatments.
2 Results
2.1 Growth performances
Growth performances are shown in Tab. 2. With the increasing of dietary canola meal levels, FI increased and CF decreased significantly. And the highest FI and the lowest CF were observed in CM100 group (P<0.05). Among the groups of FM, CM15 and CM30, there was no significant difference in final body weight, SGR and FCR (P>0.05). However, values of final body weight and SGR significantly decreased and value of FCR significantly increased in groups of CM60 and CM100 (P<0.05). No significant differences in SR, VSI and HSI were observed among all the treatments (P>0.05).
2.2 Intestine and liver histology
Histological studies of the mid intestine were performed by using scanning electron microscopy. Result showed that the intestinal villus is evenly twisted and well organized in fish from FM group (Fig. 1A). With the canola meal supplemented level increasing, the intestine villus tend to less twist and disorganized in CM60 and CM100 (Fig. 1B, 1C). However, extremely intestinal villus without twist was also observed in CM100 (Fig. 1D). Liver tissue was also affected by dietary treatment. Generally speaking, compared with FM group, distinct enlarged liver cell together with nuclear perturbations were observed in fish fed diets with canola meal replacement (Fig. 2). FM treatment had minimal vacuolation and compact hepatocytes with nuclear in the center of cells. Then, high levels of vacuolation in hepatocytes with increasing dietary canola meal replacement were observed, with nuclear located beside the cell membrane. Obvious circular vacuoles were observed in CM60 and CM100 treatment, however, the circular vacuoles in CM100 treatment are smaller that these from CM60 treatment.
2.3 Skeleton deformity
Fig. 3 and Fig. 4 showed the skeletal deformity of large yellow croaker fed with the experimental diets CM30, CM60 and CM100. Vertebral body malformations were observed. The shape of fish was related to skeletal deformity. The most common vertebral deformities found in the present study were kyphosis,lordosis, vertically shifted, compression without X-structure and widely spaced. They all were observed in CM30, CM60 and CM100 groups (Fig. 3). Several mouth deformities of large yellow croaker fed fish meal replaced by canola meal diet: elongation or lengthening of either upper or lower jaw (observed in CM60 and CM100 treatment, respectively), pugheadedness (observed in CM 60 treatment) (Fig. 4).
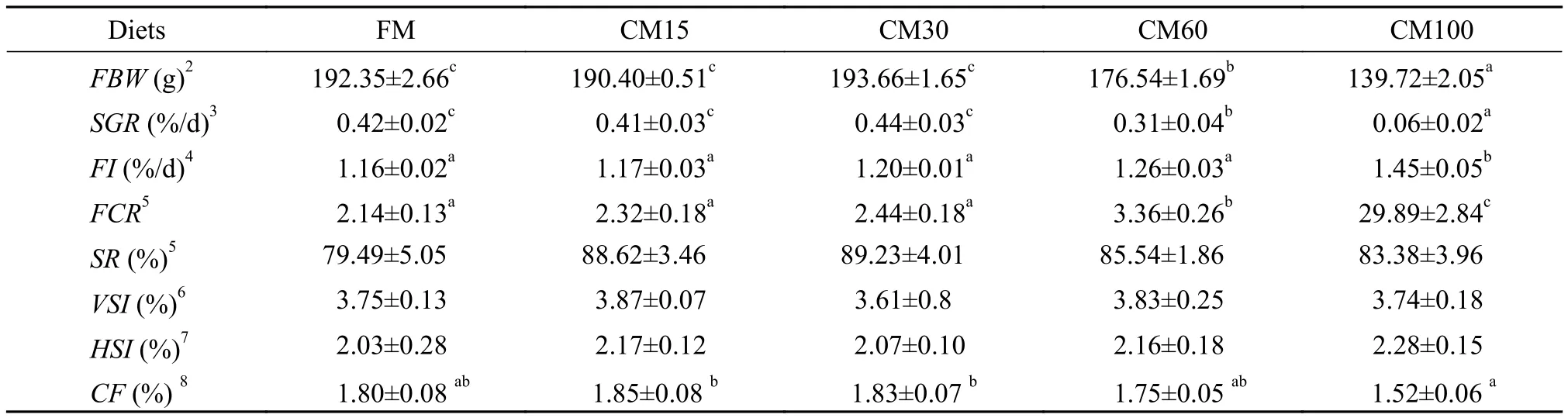
Tab. 2 Growth performance of large yellow croaker fed diets containing graded levels of fish meal replaced by canola meal for 12 weeks
2.4 Nutritional values
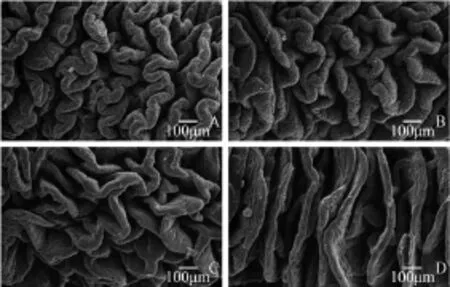
Fig. 1 Scanning electron morphology of the mid-intestine from large yellow croaker fed experiment diets FM (A), CM60 (B), CM100 (C, D) for 12 weeks
As shown in Tab. 3, the lipid content in muscle was not significantly influenced by fish meal replacement levels (P>0.05). No significant differences were observed in the contents of saturated fatty acids (SFA), mono-unsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), polyunsaturated fatty acids/saturated fatty acids (PUFA/SFA) and n-3 fatty acids (n-3) (P>0.05). Compared with the FM group, however, groups of CM30, CM60 and CM100 had significant higher levels of n-6 fatty acids (n-6) content in muscle (P<0.05). Meanwhile, groups of CM60 and CM100 had significant lower ratio of n-3 fatty acids to n-6 fatty acids (n-3/n-6) in muscle thangroups of FM and CM15 (P<0.05). The significantly higher level of docosahexaenoic acid/eicosapentaenoic acid (DHA/EPA) and arachidonic acid/eicosapentaenoic acid (ARA/EPA) were found in CM100 treatment (P<0.05).
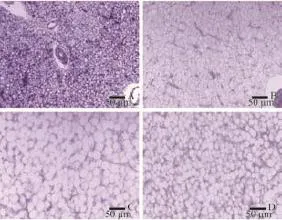
Fig. 2 Histological appearance of the liver from large yellow croaker fed experiment diets FM (A), CM30 (B), CM60 (C) and CM100 (D) for 12 weeks (×20 magnification)
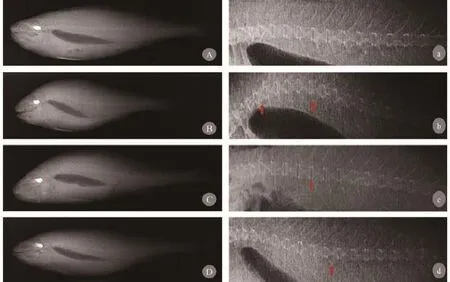
Fig. 3 Radiographs representations of vertebral body malformations in large yellow croaker fed feeds with fish meal replacement by canola meal for 12 weeksA, B, C and D illustrate the shape of fish were changed according to different kinds of skeletal deformity, while a, b, c and d is the vertebral column of each fish respectively. (A a) Vertebral column without signs of vertebra column deformations. (B b) Serious vertical shift and vertebra lost X-structure cause vertebral column deformity. (C c) Typical kyphosis with vertical shifted vertebra and enlarged intervertebral space. (D d) Typical lordosis with vertically shifted
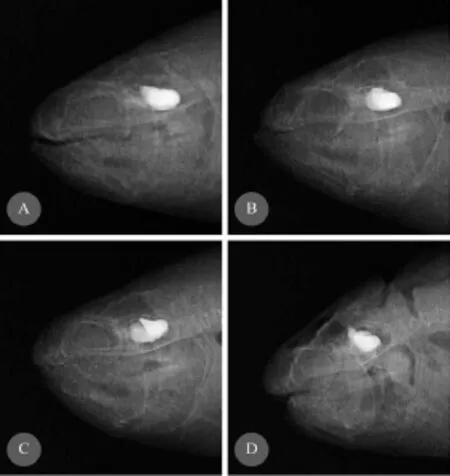
Fig. 4 Radiographs representations of mouth deformities of large yellow croaker fed feeds with fish meal replacement by canola meal for 12 weeksA. Normal mouth without signs of jaw deformations; B, C. Elongation or lengthening of either the upper or lower jaw; D. pugheadedness with a severe underdevelopment of the upper jaw, resulting in the appearance of a much lower jaw
Replacement of dietary fish meal by canola meal had no significant effects on the protein content and the amino acids composition of muscle (Tab. 4) (P>0.05).
3 Discussion
Generally in the study of dietary fish meal replacement, as the increasing of replacement levels of fish meal by alternative protein sources, additional amino acids were supplemented to balance the dietary amino acid compositions. Meanwhile, fish oil (fatty acids) was supplemented to keep the dietary lipid content constantly. In the present study, to avoid potential effects from other protein and lipid sources than canola meal, isonitrogenous or isoenergetic diets were not used. The only two variables in feed stuffs among all the experimental diets were fish meal and canola meal. The purpose was to create an extreme condition to see what really happens and the potential risks when dietary fish meal was replaced by canola meal. Results showed that the growth of large yellow croaker was significantly decreased when the replacement levels of fish meal by canola meal were higher than 30%. Meanwhile, FI increased. It could be as a compensatory mechanism of insufficient nutrients content in diets caused by canola meal replacing fish meal. In most cases, replacing fishmeal by plant protein sources reduced feed intake, which was one of the important factors for low growth performance of carnivorous fish species[19]. In the present study, it seemed that the decline of fish growth could not attribute to feed palatability since feed intake was increased with the increasing of canola meal levels in diets. This might also indicate that the attractants used in the present study could help to improve the palatably of high levels inclusion of canola meal in the feeds. It was suggested that the decline of growth was due to the lower feed utilization, lower protein and lipid contents in canola meal supplemented in the diets, or some other factors, such as digestive or metabolic system abnormalities as described below.
Growth rate was mainly determined by food utilization, in which digestion and absorption of nutrients play the first and important role, while the morphology of fish digestive system might reflects the adaptation to various diets[20]as well as the health status[21]. In the present study, morphological analysis of fish intestine was performed using scanning electron microscopy (Fig. 1), which showed that as the increasing of fish meal replacement levels, the intestine villus tended to disorganized and less twist. These could decrease absorption area and lead to dysfunction of digestion and absorption. Furthermore, the transportation and assimilation of micro particle nutrients could be hindered[22]. Eventually, it could reduce the feed utilization and threaten to the health of fish. Studies on intestinal morphology changes caused by plant protein sources revealed that there could be several reasons, such as unavailable carbohydrates[11]antinutritional factors[23]and toxic/antigenic components[24]. Although the dominating reason why canola meal caused severe damage in intestine was still unclear, results in the present study indicated that as a carnivorous fish, large yellow croaker was not adapt to high level of canola meal in diet.
The histological appearance of liver tissue was investigated in the present study with the main character of distinct circular vacuoles together with nuclear perturbations observed in fish from the groups with canola meal supplemented diets. The vacuolation of hepatogcytes in the present was likely an accumulation of lipid droplets[25]. Generally speaking, insufficient intake of nutrients which are of vital important to lipid metabolism may reduce VLDL production, thus cause abnormal lipid transportation and accumulation in liver[26,27]. According to a study in sea bass, accumulation of lipid droplets reflected thehepatic dysfunctions of nutritional origin. This phenomenon linked to disturbances in fat metabolism, more specifically, a deviation in the metabolism of fatty acids[28].Study on striped trumpeter (Latris lineata) found that low level of DHA in diets would cause highly vacuolated in liver tissue[25]. Similarly, Salhi, et al.[29]found that high numbers of lipid vacuoles in fish hepatocytes were accumulated due to low n-3 HUFA and polar lipid level in diets. In the present study, essential fatty acids composition such as DHA and EPA in both experimental diets and muscle tis-
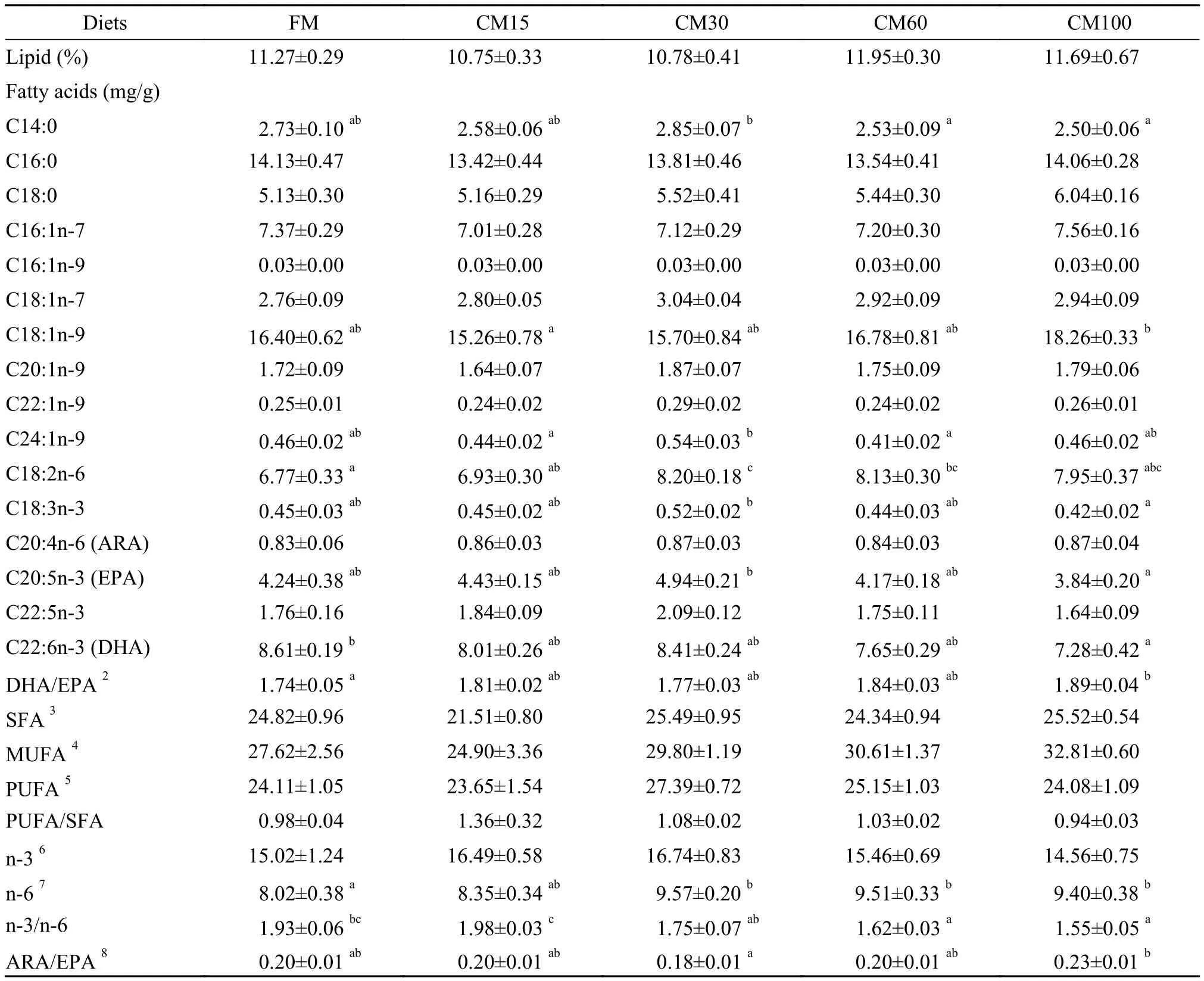
Tab. 3 Lipid content and fatty acids composition in muscle of large yellow croaker fed diets containing graded level of fish meal replaced by canola meal for 12 weeks1(wet basis)
Note: Values (means±S.E.M.) represent means of nine replicates per group. Values in each row with different superscripts have significant differences ( P <0.05);2DHA/EPA: docosahexaenoic acid/eicosapentaenoic acid (C22: 6n-3/C20: 5n-3);3SFA: saturated fatty acids include C12: 0, C13: 0 C14: 0 C15: 0 C16: 0 C18: 0 C19: 0 C20: 0 C22: 0 C23: 0 C24: 0 11-Me, C12: 0, 12-Me, C13: 0, 13-Me, C14: 0, 4, 8, 12-3Me, C13: 0, 5, 9, 13-3Me, C14: 0, 15-Me, C16: 0, 9, 10-Me, C18: 0;4MUFA: mono-unsaturated fatty acids include C14: 1n-3, C14: 1n-5, C16: 1n-7, C16: 1n-9, 7-Me, C16: 1n-6, C17: 1n-7, C18: 1n-5, C18: 1n-7, C18: 1n-9, C19: 1n-9, C20: 1n-7, C20: 1n-9, C22: 1n-9, C24: 1n-9;5PUFA: polyunsaturated fatty acids including: C18: 2n-6, C18: 2n-9, C20: 2n-6, C20: 2n-9, C18: 3n-3, C22: 3n-6, C18: 4n-3, C20: 4n-3, C20: 4n-6, C22: 4n-6, C20: 5n-3, C22: 5n-3, C22: 6n-3;6n-3: n-3 fatty acids including: C18: 3n-3, C18: 4n-3, C20: 4n-3, C20: 5n-3, C22: 5n-3, C22: 6n-3;7n-6: n-6 fatty acids including: 7-Me, C16: 1n-6, C18: 2n-6, C20: 2n-6, C22: 3n-6, C20: 4n-6, C22: 4n-6;8ARA/EPA: arachidonic acid/eicosapentaenoic acid (C20: 4n-6/C20: 5n-3) sues decreased as dietary fish meal levels decreased. It seems that the low level of fatty acids especially n-3 HUFA in the canola diets might be one of the important reasons to explain this phenomenon. Meanwhile, the circular vacuoles in CM100 treatment are smaller than those in CM60 treatment. It indicated that under the disturbed lipid metabolism condition, amount of energy intake may also contribute to the abnormal lipid droplet accumulation. Moreover, compared with fish meal, canola meal was deficient in many nutrients (e.g., essential amino acids and essential vita-mins and minerals), thus it is difficult to get a clear explanation at the moment. Further work is needed.
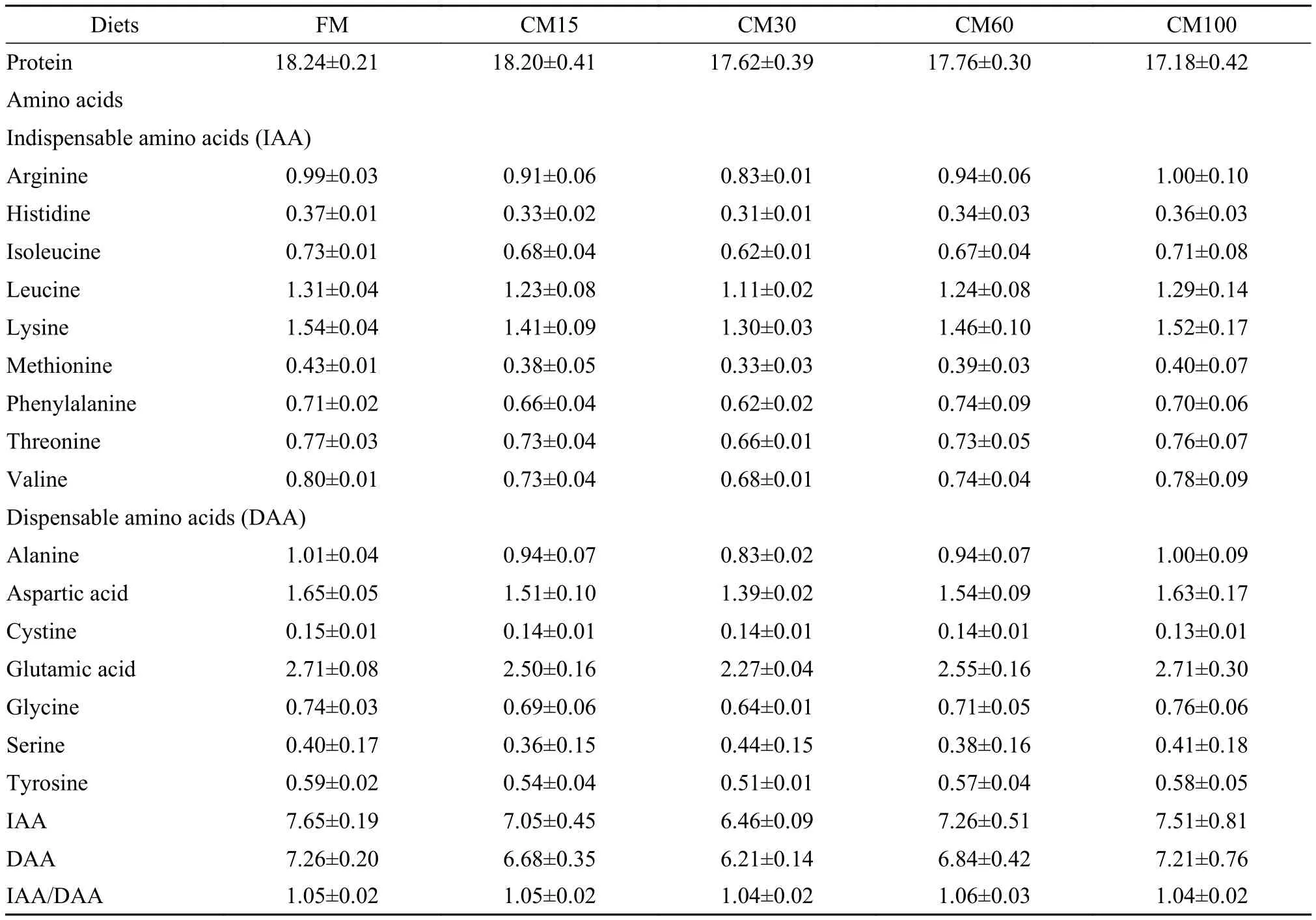
Tab. 4 Muscle protein content and amino acids composition of large yellow croaker fed diets containing graded level of fish meal replaced by canola meal for 12 weeks (%, wet basis)
Skeletal health/deformity can be detrimental to fish production performance[30]. Thus, in the present study, evaluation of potential fish health risks was also included. Vertebrae deformities (e.g., lordosis, kyphosis, compression without X-structure, vertically shifted and widely spaced) as well as jaw deformities (e.g., pug-headedness, elongation or lengthening of either the upper or lower jaw)were found in canola meal supplemented groups (CM30, CM60 and CM100). Different causative factors such as physiological, environmental, genetic, xenobiotic and nutritional factors had been found to be related to the impaired skeletal health of fish. Furthermore, among the nutritional factors, vitamins, minerals (including calcium and phosphorus)as well as lipids especially essential fatty acids were supposed to be of vital important to skeletal health[31]. The reasons that cause skeletal deformities in the present study could be complicate. Further research is still needed.
Fish is unique as healthy food for human consumption. One of the most important reasons is that it provides long chain n-3 polyunsaturated fatty acids, which can be altered by feeding[32]. In the present study, the concentrations of individual fatty acids in the lipid fraction of fish muscle showed that C18: 1n-9 presented in the greatest amount, followed by C16: 0, C22: 6n-3 (DHA), C16: 1n-7 and C18: 2n-6. To some extent, the fatty acids profile in muscle of fish reflects that in diets. In the present study, both DHA and EPA were significantly reduced in muscle in the CM100 treatment, which had similar tendency with the content of them in diets. In addition to optimal quantities of essential fatty acids, PUFA/SFA and n-6/ n-3 are considered good indexes of nutritional value[33]. Although PUFA/SFA in muscle were not significantly affected by dietary canola meal levels, n-3/n-6was extremely reduced especially when fish meal replacement level was over 60%. Similarly, n-6 content in CM100 diet (0.28) was more than doubled of that in FM diet (0.13). Besides, with the increasing of canola meal supplement, dietary crude lipid content decreased from 13% (FM) to 9.34% (CM100),which could also contribute to the decreasing level of fatty acids in muscle.
Foods containing relatively high n-3/n-6 ratio are favorable for human health[34]. From this point of view, the nutritional value of large yellow croaker fed with canola meal was reduced in the present study. However, EPA+DHA levels (from 11.12 to 13.35 mg/ g), PUFA/SFA (from 0.94 to 1.36) and n-6/n-3 (from 0.51 to 0.65) were still within the range of healthy food as recommendation for human[35,36].
In order to reveal the potential risks caused by canola meal in diets and to avoid potential effects concealed by other protein and lipid sources, graded levels of fish meal replacement by corresponding weight of canola meal were used in the present study. Inevitably, non-isonitrogenous and non-isoenergetic diets may result in fish malnutrition and uncertain reasons for the results. However, after getting rid of disturbance from other feed stuffs, taken a single plant protein as a whole could enhance our understanding of the specific plant protein especially in potential risks aspect. Results in the present study showed that higher levels (60% and 100%) of replacement fish meal by canola meal not only reduce growth performance but also cause seriously altered digestive histology profile of intestine and liver, poor skeletal health and reduced nutritional value of fish in regard of fatty acid compositions. Although the specific reasons for above phenomena are complicated, all them are attribute to the composition differences between canola meal and fish meal. To make better use of canola meal in fish feeds, it should be considered that high levels of canola meal in diets may cause organ damages of fish, leading to reduced growth performance. By balancing the nutrients in the feed, canola meal could be used better without potential risks. The present study laid the foundation for further improvement of canola meal as protein sources for fish.
4 Conclusion
The present study focused on the potential risks of high levels of fish meal replacement by canola meal on the growth, health and nutritional value of large yellow croaker. Results showed that without supplementing amino acids and oil in diets, canola meal could replace fish meal up to 30% by dry matter weight basis without significant reduced growth of large yellow croaker. Higher (60% and 100%) replacement of fish meal by canola meal had significant negative effects on the growth and health of large yellow croaker with decreased feed utilization, seriously altered histology profile of intestine and liver,
[1]Luo Z, Liu C X, Wen H. Effect of dietary fish meal replacement by canola meal on growth performance and hepatic intermediary metabolism of genetically improved farmed tilapia strain of Nile tilapia, Oreochromis niloticus, reared in fresh water [J]. Journal of the World Aquaculture Society, 2012, 43(5): 670—678
[2]Higgs D, Fegerlund U, Mcbride J, et al. Evaluation of some plant proteins in complete diets for tilapia (Sarotherodon massambicus) [J]. Aquaculture, 1983, 27(2): 97—109
[3]Bulbul M, Kader M A, Koshio S, et al. Effect of replacing fishmeal with canola meal on growth and nutrient utilization in kuruma shrimp Marsupenaeus japonicus (Bate) [J]. Aquaculture Research, 2014, 45(5): 848—858
[4]Collins S A, Desai A R, Mansfield G S, et al. The effect of increasing inclusion rates of soybean, pea and canola meals and their protein concentrates on the growth of rainbow trout: concepts in diet formulation and experimental design for ingredient evaluation [J]. Aquaculture, 2012, S344-349(2): 90—99
[5]Friedman M. Nutritional value of proteins from different food sources. A review [J]. Journal of Agricultural and Food Chemistry, 1996, 44(1): 6—29
[6]Khattab R, Arntfield S. Functional properties of raw and processed canola meal [J]. LWT-Food Science and Technology, 2009, 42(6): 1119—1124
[7]Enami H. A review of using canola/rapeseed meal in aquaculture feeding [J]. Journal of Fisheries and Aquatic Science, 2011, 6(1): 22—36
[8]Shafaeipour A, Yavari V, Falahatkar B, et al. Effects of canola meal on physiological and biochemical parameters in rainbow trout (Oncorhynchus mykiss) [J]. Aquaculture Nutrition, 2008, 14(2): 110—119
[9]Higgs D A, Mcbride J R, Markert J R, et al. Evaluation of Tower and Candle rapeseed (canola) meal and Bronowski rapeseed protein concentrate as protein supplements in practical dry diets for juvenile chinook salmon (Oncorhynchus tshawytscha) [J]. Aquaculture, 1982, 29(s 1—2): 1—31
[10]Cheng Z, Ai Q, Mai K, et al. Effects of dietary canola meal on growth performance, digestion and metabolism of Japanese seabass, Lateolabrax japonicas [J]. Aquaculture, 2010, poor skeletal health and reduced nutritional value of fish in regard of fatty acid compositions. However, these changes didn't influence the fact that large yellow croaker was also recommended as a healthy food fish.
Acknowledgements:
The authors would like to thank Yi Xin-Wen, Li Jun, Gu Zhi-Xiang, Feng Lei-Li, Tang Zhuo-Yi, Zhang Han-Le, Cao Juan-Juan, Mu Hua and Ma Jun for their kind supports.
[11]Cai C, Song L, Wang Y, et al. Assessment of the feasibility of including high levels of rapeseed meal and peanut meal in diets of juvenile crucian carp (Carassius auratus gibelio♀× Cyprinus carpio♂): Growth, immunity, intestinal morphology, and microflora [J]. Aquaculture, 2013, 410-411: 203—215
[12]Fishery Bureau, Ministry of Agriculture. China Fishery Statistical Yearbook [M]. Beijing: China Agriculture Press. 2015, 28
[13]Yi X W, Li J, Xu W, et al. Effects of dietary xanthophylls/ astaxanthin ratios on the growth and skin pigmentation of large yellow croaker Larimichthys crocea (Richardson, 1846) [J]. Journal of Applied Ichthyology, 2015, 31(4): 780—786
[14]AOAC. Official Methods of Analysis, 16th edn [M]. Association of Official Analytical Chemists, Arlington, VA, USA. 1995, 1298
[15]Folch J, Lees M, Sloane-Stanley G. A simple method for the isolation and purification of total lipids from animal tissues [J]. Journal of Biological Chemistry, 1957, 226(1): 497—509
[16]Taha A Y, Metherěl A H, Stark K D. Comparative analysis of standardized and common modifications of methods for lipid extraction for the determination of fatty acids [J]. Food Chemistry, 2012, 134(1): 427—433
[17]Boglione C, Gagliardi F, Scardi M, et al. Skeletal descriptors and quality assessment in larvae and post-larvae of wildcaught and hatchery-reared gilthead sea bream (Sparus aurata L. 1758) [J]. Aquaculture, 2001, 192(1): 1—22
[18]Witten P E, Gil-Martens L, Huysseune A, et al. Towards a classification and an understanding of developmental relationships of vertebral body malformations in Atlantic salmon (Salmo salar L.) [J]. Aquaculture, 2009, 295(1—2): 6—14
[19]Hill H A, Trushenski J T, Kohler C C. Utilization of soluble canola protein concentrate as an attractant enhances production performance of sunshine bass fed reduced fish meal, plant-based diets [J]. Journal of the World Aquaculture Society, 2013, 44(1): 124—132
[20]Omnes M H, Silva F C P, Moriceau J, et al. Influence of lupin and rapeseed meals on the integrity of digestive tract and organs in gilthead seabream (Sparus aurata L.) and goldfish (Carassius auratus L.) juveniles [J]. Aquaculture Nutrition, 2015, 21(2): 223—233
[21]Walker W. Exogenous nucleotides and gastrointestinal immunity [C]. Transplantation Proceedings. 1996, 28(5): 2438—2441
[22]Wang C, Zhu X, Han D, et al. Responses to fishmeal and soybean meal-based diets by three kinds of larval carps of different food habits [J]. Aquaculture Nutrition, 2014, 21(5): 552—568
[23]Van Den Ingh T, Krogdahl, Olli J, et al. Effects of soybeancontaining diets on the proximal and distal intestine in Atlantic salmon (Salmo salar): a morphological study [J]. Aquaculture, 1991, 94(4): 297—305
[24]Bakke-Mckellep A, Press C M, Baeverfjord G, et al. Changes in immune and enzyme histochemical phenotypes of cells in the intestinal mucosa of Atlantic salmon, Salmo salar L., with soybean meal-induced enteritis [J]. Journal of Fish Diseases, 2000, 23(2): 115—127
[25]Bransden M, Cobcroft J, Battaglene S, et al. Dietary 22: 6n-3 alters gut and liver structure and behaviour in larval striped trumpeter (Latris lineata) [J]. Aquaculture, 2005, 248(1): 275—285
[26]Tocher D R, Bendiksen E, Campbell P J, et al. The role of phospholipids in nutrition and metabolism of teleost fish [J]. Aquaculture, 2008, 280(1): 21—34
[27]Espe M, Rathore R M, Du Z Y, et al. Methionine limitation results in increased hepatic FAS activity, higher liver 18: 1 to 18: 0 fatty acid ratio and hepatic TAG accumulation in Atlantic salmon, Salmo salar [J]. Amino Acids, 2010, 39(2): 449—460
[28]Mosconi-Bac N. Hepatic disturbances induced by an artificial feed in the sea bass (Dicentrarchus labrax) during the first year of life [J]. Aquaculture, 1987, 67(1): 93—99
[29]Salhi M, Hernández-Cruz C M, Bessonart M, et al. Effect of different dietary polar lipid levels and different n-3 HUFA content in polar lipids on gut and liver histological structure of gilthead seabream (Sparus aurata) larvae [J]. Aquaculture, 1999, 179(1—4): 253—263
[30]Noble C, Jones H a C, Damsgård B, et al. Injuries and deformities in fish: their potential impacts upon aquacultural production and welfare [J]. Fish Physiology and Biochemistry, 2012, 38(1): 61—83
[31]Lall S P, Lewis-Mccrea L M. Role of nutrients in skeletal metabolism and pathology in fish - An overview [J]. Aquaculture, 2007, 267(1—4): 3—19
[32]Lie. Flesh quality-the role of nutrition [J]. Aquaculture Research, 2001, 32(s1): 341—348
[33]Steffens W, Wirth M. Freshwater fish-an important source of n-3 polyunsaturated fatty acids: a review [J]. Archives of Polish Fisheries, 2005, 13(1): 5—16
[34]Simopoulos A P. Omega-3 fatty acids in inflammation and autoimmune diseases [J]. Journal of the American College of Nutrition, 2002, 21(6): 495—505
[35]Authority EFS. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol [J]. EFSA Journal, 2010, 8(3): 1461
[36]Rebole A, Velasco S, Rodriguez M L, et al. Nutrient content in the muscle and skin of fillets from farmed rainbow trout (Oncorhynchus mykiss) [J]. Food Chemistry, 2015, 174: 614—620
305(1): 102—108
双低菜粕高水平替代饲料鱼粉对大黄鱼潜在风险的评估:生长、健康和营养价值
孟玉琼1苗 新1孙瑞健2马 睿3申屠基康4张文兵1麦康森1
(1. 中国海洋大学水产学院,农业部水产动物营养与饲料重点实验室,海水养殖教育部重点实验室,青岛 266003; 2. 通威股份有限公司技术中心, 成都 610041; 3. 青海大学生态环境工程学院,西宁 810016; 4. 宁波市海洋与渔业研究院,宁波 315010)
研究从生长、健康和营养价值方面评估了高水平的双低菜粕替代饲料鱼粉对大黄鱼潜在的危害。在鱼粉含量60%的基础饲料(FM)上按照质量分数用双低菜粕分别替代15% (CM15)、30% (CM30)、60% (CM60)和100% (CM100)的鱼粉, 配制成5种实验饲料。每种饲料投喂5个网箱的大黄鱼[初重(135.38±1.02) g], 即每个处理5个重复, 进行12周的养殖实验。结果表明, 当双低菜粕替代水平在15%和30%时, 大黄鱼的生长及饲料系数并没有受到显著性的影响。然而, 当替代水平高于30%时, 大黄鱼的末重和特定生长率均显著降低, 而饲料系数显著升高(P<0.05)。当替代水平达到100%时, 大黄鱼摄食率达到最高值而肥满度达到最低值(P<0.05)。在组织形态方面, 大黄鱼摄食双低菜粕替代的饲料后肠道绒毛的弯曲程度减少并且排列更加不规则, 而肝细胞则呈现出圆形空泡状并伴随着细胞核的偏移。对大黄鱼骨骼进行X-射线扫描发现, 摄食双低菜粕的大黄鱼椎体和头部出现了畸形。在营养价值方面, 双低菜粕替代鱼粉并未显著影响大黄鱼背肌的脂肪含量、蛋白含量和氨基酸组成, 然而脂肪酸组成受到了显著影响, 即N-6系列脂肪酸含量显著升高, 而DHA与EPA含量显著降低(P<0.05)。根据欧洲食品安全局 (EFSA)的相关标准, 这些营养价值的变化并没有影响大黄鱼作为健康食品的功能。由此可见, 高水平(60% 和100%)的双低菜粕替代鱼粉对大黄鱼的负面影响主要表现为降低大黄鱼的生长性能、改变肠道和肝脏组织形态, 以及影响大黄鱼的骨骼健康。然而, 双低菜粕替代鱼粉养殖大黄鱼的肌肉仍然符合人类的膳食要求。因此, 双低菜粕替代鱼粉并没有影响大黄鱼作为食用鱼的营养价值。
大黄鱼; 双低菜粕; 鱼粉; 生长; 健康; 营养价值
S965.3 Document code: A Article ID: 1000-3207(2017)01-0127-12
10.7541/2017.17
Received date: 2016-03-08; Accepted date: 2016-06-17
Foundation item: Supported by the National Natural Science Foundation of China (No. 31372542); the State Spark-Program China (2014GA701001); the Fundamental Research Funds for the Central Universities, Ocean University of China (No. 201562017)
Brief introduction of author: Meng Yu-Qiong, Female, Ph.D; Engaged in fish nutrition and feed research. E-mail: yuqiongcheer@163.com
Zhang Wen-Bing, E-mail: wzhang@ouc.edu.cn

