温州市PM2.5中水溶性离子污染特征及来源分析
葛琳琳,郑元铸,涂圣锋,朱京科,王俏丽,王向前,李素静*,李 伟,
(1.浙江大学 环境工程研究所,浙江 杭州 310058; 2.温州市环境监测中心站 浙江 温州 325003; 3.浙江大学 生物质
温州市PM2.5中水溶性离子污染特征及来源分析
葛琳琳1,郑元铸2,涂圣锋2,朱京科3,王俏丽4,王向前3,李素静3*,李 伟1,3
(1.浙江大学 环境工程研究所,浙江 杭州 310058; 2.温州市环境监测中心站 浙江 温州 325003; 3.浙江大学 生物质
化工教育部重点实验室 工业生态与环境研究所,浙江 杭州 310027; 4.浙江大学 热能工程研究所,浙江 杭州 310027)

温州;PM2.5;水溶性离子;污染特征;源解析
Characteristics and sources apportionment of water-soluble ions in PM2.5of Wenzhou, Zhejiang Province. Journal of Zhejiang University(Science Edition), 2017,44(1):112-119
0 引 言

温州市位于浙江省东南沿海地区,产业特色为服装、电器、制鞋、制革、汽摩配和印刷包装等,是浙江省经济发展较快的城市之一.随着城市化进程的加快以及工业的迅速发展,灰霾、酸雨和光化学污染等大气污染问题日益凸显,亦越来越受关注.据温州市2014年环境状况公报,其大气首要超标污染物为PM2.5,全市平均霾日数为62.5 d,比2013年减少33%(30 d).目前,还没有关于温州市大气PM2.5中水溶性离子的研究.为此,本研究在温州城区设置4个采样点,分4个季度采集PM2.5样品,研究温州市大气PM2.5中水溶性离子的污染特征和主要来源,对了解温州市大气复合污染特征、制定有效的防治对策具有重要意义.
1 材料与方法
1.1 样品采集
为全面评价温州主城区(鹿城区、瓯海区和龙湾区)大气细颗粒物的污染现状,根据《环境空气颗粒物源解析监测技术方法指南(试行)》(以下简称指南)的规定,在温州市区范围内布设4个国控(或省控)监测点位,温州市环境监测中心站(简称市站)、温州市环境监测中心站瓯海分站(简称瓯海)、龙湾区环境空气监测点(简称龙湾)以及南浦环境空气监测点(简称南浦)进行环境受体样品采集.4个采样点均位于温州市主城区.市站点位于鹿城区,南浦和瓯海点位于瓯海区,龙湾点位于龙湾区;市站采样点位于瓯江边,周围是学校和居民区,大气污染来源相对较少;南浦采样点位于温州大道和南浦路交汇处,2条道路均是双向六车道的市区交通干道,平时车流量大;瓯海附近住宅区密集,紧邻交通干道,车流量较大,且附近有一些较大的货运公司;龙湾点位于瓯江附近的居民区,周边有一些机械阀门铸造企业和混凝土企业.4个站点同步采集得到的环境受体样品能够代表温州市区空气中PM2.5的来源状况.具体监测点分布见图1.
根据指南中对采样时间及周期的规定,本项目在充分研究温州市颗粒物浓度、排放源的季节性变化特征和气象因素后,分别于2015年1月4~12日(冬季)、4月16~25日(春季)、7月22~29日(夏季)和10月18~24日(秋季)对4个采样点进行同步PM2.5采样.使用崂应2030型智能TSP中流量采样器采样,设置采样流量为100 L·min-1.采集的样品量应满足分析要求且不过载,设置采样时间为20 h.采样滤膜为石英纤维滤膜(QMA 1851-090型,直径90 mm).
崂应2030型智能TSP中流量采样器技术参数:
采样流量:100 L·min-1,分辨率0.1 L·min-1,准确度≤±5.0%,流量相对标准偏差≤2.0%;
计前温度:-30~99 ℃,分辨率0.1 ℃,准确度≤±1.0 ℃;
大气压:70~130 kPa,分辨率0.01 kPa,准确度≤±500 Pa;
切割特性:PM10采样头Da50=(10±0.5)μm,g≤1.5±0.1;PM2.5采样头Da50=(2.5±0.2)μm,g≤1.2±0.1;
入口速度0.3 m·s-1,有效滤膜直径Φ 80 mm,连接头M20×1.5,噪声≤59 dB(A),工作电源AC220V±10%,50Hz;

图1 温州市PM2.5监测点位分布示意Fig.1 Sampling sites of PM2.5 in Wenzhou City
执行标准:HJ 618-2011《环境空气PM10和PM2.5的测定重量法》,HJ/T 93-2013《环境空气颗粒物(PM10和PM2.5)采样器技术要求及检测方法》.
1.2 膜处理
在样品采集前,将装有石英膜的铝箔袋敞口放到马弗炉中,在450 ℃条件下灼烧4 h,待石英膜自然冷却,取出后将铝箔袋密封.另外,用于保存石英膜膜盒的盒内铝箔也需在450 ℃灼烧4 h,且为避免镊子直接接触石英膜,滤膜准备过程中使用的镊子需用以上铝箔包好镊子尖头与膜接触的部分.待样品采集后,将滤膜放入膜盒密封、编号,放入-30 ℃的冰箱内冷冻保存.采样前后滤膜均放在恒温恒湿箱内平衡24 h以上,恒重条件设为温度(20±1) ℃、相对湿度(50±5)%RH.平衡后用万分之一分析天平称重.
1.3 样品预处理及分析
将滤膜剪成约0.5 cm×3 cm的条状放入带塞平口试管中,加入20.00 mL超纯水,将试管置于超声仪中超声震荡30 min后静置,取上层清液.上层清液经0.22 μm针式过滤头过滤后,用ICS-1100离子色谱(美国Dionex公司)对滤液中的水溶性离子进行分析.

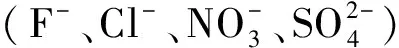
2 结果与讨论
2.1 PM2.5中水溶性离子浓度


表1 温州市2015年PM2.5中主要水溶性离子浓度及占比
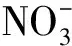

表2 不同城市大气PM2.5中主要水溶性离子浓度
注 BDL表示数值低于检测限.
2.2 PM2.5中水溶性离子时空变化特征


图2 2015年观测期间温州市区PM2.5中水溶性离子的时空分布Fig.2 Spatial and temporal distribution of water-soluble ions in PM2.5 in Wenzhou during 2015A曲硝酸根硫酸根铵根氯(Cl-) E其他水溶性离子
2.3 阴阳离子平衡

阳离子=

(1)

(2)

图3 水溶性阴阳离子的相关性Fig.3 Correlations between anions and cations
温州市阴离子/阳离子的年均值为1.01±0.11,对温州市区PM2.5中水溶性离子做线性回归分析,发现阴阳离子存在明显相关性,R2为0.78,平衡方程斜率大于1.由此可见,温州市区PM2.5总

2.4 PM2.5中水溶性离子相关性分析


表3 水溶性离子与PM2.5 Pearson相关系数
** 在0.01水平(双侧)上显著相关; * 在0.05水平(双侧)上显著相关.




(3)

(4)

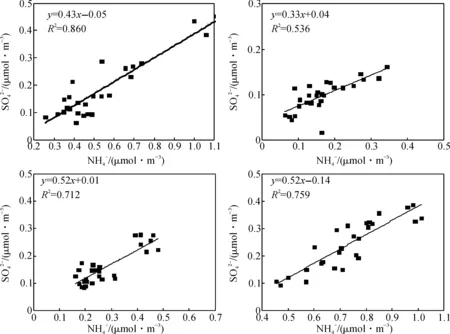
图和的相关性Fig.4 Correlations between and
较低时,有利于颗粒物中NH4NO3的形成.另外,也有研究表明,NOR与光照强度、大气中氧化自由基、H2O2以及臭氧等有关[37].如表4所示,温州市区四季NOR平均值均大于0.1,年均值为0.13±0.04,说明存在二次转化.冬、春两季NOR值相对较高,可能主要受到温度的影响.

表4 PM2.5中SOR和NOR的水平
2.6 主成分分析
采用主成分分析法(principal component analysis, PCA)对温州市PM2.5样品中水溶性离子进行源解析,从而对PM2.5中水溶性离子的来源做定性分析.主成分分析采用SPSS 19.0完成,分析结果见表5.

表5 因子载荷矩阵
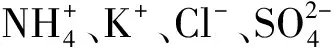
温州市包含4家主要的燃煤发电厂(浙能乐清电厂、浙能温州电厂、温州特鲁莱电厂以及华润电力),主要工业为合成革、塑料、服装、电器、化工、化纤、印染和造纸等,虽然工业燃烧用煤量远小于燃煤发电厂,但工业燃烧不够完全,污染物去除效率远低于火力发电,因此,工业燃煤污染对环境空气的贡献也较大.根据温州市国民经济和社会发展统计公报,2015年年末全市户籍总人口811.21万,其中市区人口165.93万;年末温州市机动车保有量202.68万辆,较2014年年末增加14.45万辆;全市房屋施工面积4.650 82×107m2;全市粮食播种面积为12.294×104hm2(184.41万亩);国民经济三次产业结构的比重分别为2.7%(第一产业)、45.5%(第二产业)和51.8%(第三产业).根据主成分分析结果,结合温州市实际情况,温州市PM2.5中水溶性离子主要来源于燃煤(包括火力发电和工业燃烧过程)、机动车尾气、生物质燃烧以及道路和建筑扬尘,另外颗粒物中F-主要来源于纺织、造纸和涂料等行业.
3 结 论


3.3 温州市SOR和NOR的年均值分别为0.44±0.09和0.13±0.04,且夏秋季SOR大于春冬季,NOR小于春冬季.
3.4 根据主成分分析结果,结合实际情况,可知温州市PM2.5中水溶性离子主要来源于燃煤(包括火力发电和工业燃烧过程)、机动车尾气、生物质燃烧以及道路和建筑扬尘,另外颗粒物中F-主要来源于纺织、造纸和涂料等行业.
[1] DOMINICI F, PENG R D, BELL M L, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases[J]. Jama-Journal of the American Medical Association,2006,295(10):1127-1134.
[2] LIU Y J, ZHANG T T, LIU Q Y, et al. Seasonal variation of physical and chemical properties in TSP, PM10and PM2.5at a roadside site in Beijing and their influence on atmospheric visibility[J]. Aerosol And Air Quality Research,2014,14(3):954-969.
[3] 花丛,张碧辉,张恒德.2013年1-2月华北雾、霾天气边界层特征对比分析[J].气象,2015,41(9):1144-1151. HUA C, ZHANG B H, ZHANG H D. Analysis on boundary layer characteristics in fog and haze processes in North China from January to February 2013[J]. Meteorological Monthly,2015,41(9):1144-1151.
[4] 李粟,苗海斌,康富华.石家庄市春季PM10和PM2.5浓度及其水溶性离子组分特征分析[J].河北工业科技,2015,32(1):90-94. LI S, MIAO H B, KANG F H. Analysis of mass concentration and water soluble ionic components characteristics of PM10and PM2.5in spring in Shijiazhuang City[J]. Hebei Journal of Industrial Science and Technology,2015,32(1):90-94.
[5] YAO X H, CHAN C K, FANG M, et al. The water-soluble ionic composition of PM2.5in Shanghai and Beijing, China[J]. Atmospheric Environment,2002,36:4223-4234.
[6] HUEGLIN C, GEHRIG R, BALTENSPERGER U, et al. Chemical characterisation of PM2.5, PM10and coarse particles at urban, near-city and rural sites in Switzerland[J]. Atmospheric Environment,2005,39(4):637-651.
[7] ANDREAE MO, SCHMID O, YANG H, et al. Optical properties and chemical composition of the atmospheric aerosol in urban Guangzhou, China[J]. Atmospheric Environment,2008,42(25):6335-6350.
[8] LAI S C, ZOU S C, CAO J J, et al. Characterizing ionic species in PM2.5and PM10in four Pearl River Delta Cities, South China[J]. Journal of Environmental Sciences,2007,19(8):939-947.
[9] 毛敏娟,孟燕军,齐冰.杭州等城市大气污染特性研究[C]//2015年中国环境科学学会学术年会.深圳:中国环境科学学会,2015:3227-3236. MAO M J, MENG Y J, QI B. Atmospheric pollution characteristics research of Hangzhou[C]// Academic Annual Meeting of Chinese Society for Environmental Sciences in 2015. Shenzhen: Chinese Society for Environmental Sciences,2015:3227-3236.
[10] 杨懂燕,刘保献,张大伟,等.2012~2013年间北京市PM2.5中水溶性离子时空分布规律及相关性分析[J].环境科学,2015,36(3):768-773. YANG D Y, LIU B X, ZHANG D W, et al. Correlation, seasonal and temporal variation of water-souble ions of PM2.5in Beijing during 2012 to 2013[J]. Environmental Science,2015,36(3):768-773.
[11] 邹亚娟,金承钰,舒加乐,等.上海春季PM2.5和PM1.0水溶性无机离子含量特征[J].实验室研究与探索,2015,34(1):44-47. ZOU Y J, JIN C Y, SHU J J, et al, Characterization of water-soluble ions in PM2.5and PM1.0of Shanghai during spring[J]. Research and Exploration in Laboratory,2015,34(1):44-47.
[12] 孙韧,张文具,董海燕,等.天津市PM10和PM2.5中水溶性离子化学特征及来源分析[J].中国环境监测,2014,30(2):145-150. SUN R, ZHANG W J, DONG H Y, et al. Chemical character and source analysis of water-soluble irons in PM10and PM2.5in Tianjin city[J]. Environmental Monitoring in China,2014,30(2):145-150.
[13] 曲健,李晶,张晶,等.沈阳市城区采暖期PM2.5中水溶性离子的化学特征[J].中国环境监测,2015,31(5):57-60. QU J, LI J, ZHANG J, et al. Chemica character of water-soluble ions in PM2.5in Shenyang city during heating season[J]. Environmental Monitoring in China,2015,31(5):57-60.
[14] KIM Y J, LEE H L, PARK S S, et al. Source identification of PM2.5particles measured in Gwangju, Korea[J]. Atmospheric Research,2008,88(3/4):199-211.
[15] YAN J P, CHEN L Q, LIN Q, et al. Chemical characteristics of submicron aerosol particles during a long-lasting haze episode in Xiamen, China[J]. Atmospheric Environment,2015,113:118-126.
[16] YANG F, TAN J, ZHAO Q, et al. Characteristics of PM2.5speciation in representative megacities and across China[J]. Atmospheric Chemistry and Physics,2011,11(11):5207-5219.
[17] CHEN J S, HE C, YIN L Q, et al. Seasonal variations and chemical compositions of PM2.5aerosol in the urban area of Fuzhou, China[J]. Atmospheric Research,2012,104:264-272.
[18] 陈璞珑,王体健,胡忻,等.南京市细颗粒物来源解析研究[J].南京大学学报:自然科学版,2015,51(3):524-534. CHEN P L, WANG T J, HU X, et al. A study of chemical mass balance source apportionment of fine particulate matter in Nanjing[J]. Journal of Nanjing University:Natural Sciences,2015,51(3):524-534.
[19] 李友平,周洪,张智胜,等.成都市城区PM2.5中二次水溶性无机离子污染特征[J].环境科学,2014,35(12):4439-4445. LI Y P, ZHOU H, ZHANG Z S, et al. Pollution characteristics of secondary water-soluble inorganic ions of PM2.5in urban Chengdu, China[J]. Environmental Science,2014,35(12):4439-4445.
[20] TAO J, ZHANG L M, HO K F, et al. Impact of PM2.5chemical compositions on aerosol light scattering in Guangzhou-The largest megacity in South China[J]. Atmospheric Research,2014,135:48-58.
[21] CHENG Y, CAO J J, HAI X, et al. Chemically-speciated on-road PM2.5motor vehicle emission factors in Hong Kong[J]. Science of the Total Environment,2010,408(7):1621-1627.
[22] QIN Y J, KIM E, HOPKE P K.The concentrations and sources of PM2.5in metropolitan New York city[J]. Atmospheric Environment,2006,40(Supp):312-332.
[23] ZHANG T, CAO J J, TIE X X, et al. Water-soluble ions in atmospheric aerosols measured in Xi’an, China: Seasonal variations and sources[J]. Atmospheric Research,2011,102:110-119.
[24] 陈诚,陈辰,汤莉莉,等.江苏沿江城市PM10和PM2.5中水溶性离子特征及来源分析[J].环境化学,2014,33(12):2123-2135. CHEN C, CHEN C, TANG L L, et al. Characteristics and sources analysis of water-soluble ions in PM10and PM2.5in cities along the Yangtze River of Jiangsu Province[J]. Environmental Chemistry,2014,33(12):2123-2135.
[25] ZHANG F W, XUA L L, CHEN J S, et al. Chemical characteristics of PM2.5during haze episodes in the urban of Fuzhou, China[J]. Particuology,2013,11:264-272.
[26] MD F K, YUICHIRO S, KOICHIRO H, et, al. Characterization of PM2.5, PM2.5-10and PMN10in ambient air, Yokohama, Japan[J].Atmospheric Research,2010,96:159-172.
[27] REMOUNDAKI E, KASSOMENOS P, MANTAS E, et al. Composition and mass closure of PM2.5in urban environment (Athens, Greece)[J].Aerosol and Air Quality Research,2013,13:72-82.
[28] SHEN Z X, LI X X, DU N, et al. Seasonal variations and evidence for the effectiveness of pollution controls on water soluble inorganic species in total suspended particulates and fine particulate matter from Xi’an, China[J]. Journal of the Air & Waste Management Association,2008,58(12):1560-1570.
[29] ZHANG F W, XU L L, CHEN J S, et al. Chemical compositions and extinction coefficients of PM2.5in peri-urban of Xiamen, China, during June 2009 to May 2010[J]. Atmospheric Research,2012,106:150-158.
[30] HAN T T, LIU X J, ZHANG Y H, et al. Role of secondary aerosols in haze formation in summer in the megacity Beijing[J]. Journal of Environmental Sciences,2015,31:51-60.
[31] LI X R, WANG L L, JI D S, et al. Characterization of the size-segregated water-soluble inorganic ions in the Jing-Jin-Ji urban agglomeration: Spatial/temporal variability, size distribution and sources[J]. Atmospheric Environment,2013,77:250-259.
[32] VOUTSA D, SAMARA C, MANOLI E, et al. Ionic composition of PM2.5at urban sites of northern Greece: Secondary inorganic aerosol formation[J]. Environmental Sciences Pollution Research,2014,21:4995-5006.
[33] BEHERA S N, SHARMA M. Investigating the potential role of ammonia in ion chemistry of fine particulate matter formation for an urban environment[J]. Science of the Total Environment,2010,408:3569-3575.
[34] GAO X M, YANG L X, CHENG S H, et al. Semi-continuous measurement of water-soluble ions in PM2.5in Jinan, China: Temporal variations and source apportionments[J]. Atmospheric Environment,2011,45:6048-6056.
[35] ZHAO X J, ZHAO P S, XU J. Analysis of a winter regional haze event and its formation mechanism in the North China Plain[J]. Atmospheric Chemistry and Physics,2013,13:5685-5696.
[36] LIU X G, SUN K, QU Y, et al. Secondary formation of sulfate and nitrate during a haze episode in megacity Beijing, China[J]. Aerosol and Air Quality Research,2015,15:2246-2257.[37] DU H H, KONG L D, CHENG T T, et al. Insights into summertime haze pollution events over Shanghai based on online water-soluble ionic composition of aerosols[J]. Atmospheric Environment,2011,45:5131-5137.
[38] TAO J, ZHANG L M, ENGLING G, et al. Chemical composition of PM2.5in an urban environment in Chengdu, China:Importance of springtime dust storms and biomass burning[J]. Atmospheric Research,2013,122:270-283.
[39] LI L, WANG W, FENG J L, et al. Composition, source, mass closure of PM2.5aerosols for four forests in Eastern China[J]. Journal of Environmental Sciences,2010,22(3):405-412.
[40] 何鹏飞,张鸿,李静,等.深圳市大气中全氟化合物的残留特征[J].环境科学,2016,37(4):1240-1247. HE P F, WANG H, LI J, et al. Residue characteristics of perfluorinated compounds in the atmosphere of Shenzhen[J]. Environmental Science,2016,37(4):1240-1247.
[41] SPINDLER G, BRUGGEMANN E, GNAUK T, et al. A four-year size-segregated characterization study of particles PM10, PM2.5and PM1depending on air mass origin at Melpitz[J]. Atmospheric Environment,2010,44:164-173.
GE Linlin1, ZHENG Yuanzhu2, TU Shengfeng2, ZHU Jingke3, WANG Qiaoli4, WANG Xiangqian3, LI Sujing3, LI Wei1,3
(1.InstituteofEnvironmentalEngineering,ZhejiangUniversity,Hangzhou310058,China; 2.WenzhouEnvironmentalMonitoringCenter,Wenzhou325003,China; 3.BiomassChemicalIndustryMinistryofEducationKeyLaboratory,InstituteofIndustrialEcologyandEnvironment,ZhejiangUniversity,Hangzhou310027,China; 4.InstituteofThermalPowerEngineering,ZhejiangUniversity,Hangzhou310027,China)
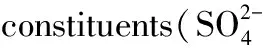
Wenzhou; PM2.5; water-soluble ions; pollution characteristics; source apportionment

2016-03-14.
葛琳琳(1991-),ORCID:http//orcid.org/0000-0002-8908-4292,女,硕士研究生,主要从事PM2.5源解析研究, E-mail: gll920@zju.edu.cn.
*通信作者,ORCID:http://orcid.org/0000-0002-7757-3881,E-mail:sujing-li@zju.edu.cn.
10.3785/j.issn.1008-9497.2017.01.016
X 51
A
1008-9497(2017)01-112-09
——浙江省温州市平阳县中心小学

