Preparation and evaluation of alpha-mangostin solid self-emulsifying drug delivery system
,Pimchnok Spsuphn,Rtt Wongsirikul, Stit Puttipiptkhchorn,b,
aDepartment of Manufacturing Pharmacy,Faculty of Pharmacy,Mahidol University,Bangkok 10400,Thailand bCenter of Excellence in Innovative Drug Delivery and Nanomedicine,Faculty of Pharmacy,Mahidol University, Bangkok 10400,Thailand
Preparation and evaluation of alpha-mangostin solid self-emulsifying drug delivery system
Kun Sodaleea,Pimchanok Sapsuphana,Ratta Wongsirikula, Satit Puttipipatkhachorna,b,*
aDepartment of Manufacturing Pharmacy,Faculty of Pharmacy,Mahidol University,Bangkok 10400,ThailandbCenter of Excellence in Innovative Drug Delivery and Nanomedicine,Faculty of Pharmacy,Mahidol University, Bangkok 10400,Thailand
A R T I C L E I N F O
Article history:
Available online 24 November 2015
Alpha-mangostin
Solid self-emulsifying drug delivery system
Solubility
Solid carrier
Alpha-mangostin(AMG),a natural xanthone extracted from Garcinia mangostana Linn,has a variety of pharmacological therapeutic effects such as antioxidant activity,antibacterial activity, anticancer,and anti-infammatory[1].However,it has poor aqueous-solubility and dissolution,which results in low bioavailability.Solid self-emulsifying drug delivery system(solid-SEDDS),an effective pharmaceutical strategy,offers the potential for enhancing the oral bioavailability of poorly water-soluble drugs[2].Therefore,solid-SEDDS is of interest as a potential method for enhancing the solubility and dissolution of AMG. The aim of this study was to develop and evaluate a solid selfemulsifying drug delivery system(solid-SEDDS)containingAMG for enhancing its solubility and dissolution.
Solubility of AMG in water,and various excipients including oil,surfactant and co-surfactant were determined at 30°C for 72 hours and the results showed that all excipients could enhance the solubility of AMG when compared to its aqueous solubility.Pseudo-ternary phase diagram was used to determine the self-emulsifying existence area.The liquid-SEDDS was formulated using Captex 200P/Tween 80/Capryol90(20/70/10 w/w/w)as oil,surfactant,and co-surfactant,respectively.After reconstituted with water,the droplet size of liquid-SEDDS was determined by photon correlation spectroscopy(PCS)and the results showed that the average droplet size of liquid-SEDDS and AMG-loaded liquid-SEDDS were 59.28±3.54 nm and 106.9±24.3 nm,respectively.Moreover,no effect of reconstituted volume of water on droplet size of AMG-loaded liquid-SEDDS was observed,indicating that it was robust to dilution.
After that,the liquid-SEDDS was converted to solid form by adsorption on two types of silica(Aeroperl 300 and Sylysia 350)(Fig.1).It was found that the solid-SEDDS with Aeroperl 300 showed better performance in powder fowability than the solid-SEDDS with Sylysia 350.Solid state characterizations of the solid-SEDDS performed by differential scanning calorimetry(DSC)and powder X-ray diffraction(PXRD)suggested that AMG in the solid-SEDDS was in the amorphous or molecular dispersion state.The comparison of dissolution profles in simulated gastric fuid(SGF)without pepsin showed that the solid-SEDDSs withAeroperl 300 and Sylysia 350 released 18.82% and 7.71%of AMG within 60 minutes,respectively.Whereas, intact AMG powder dissolved only 0.26%.In conclusion,the solid-SEDDS is a promising method to enhance the solubility and dissolution of AMG.
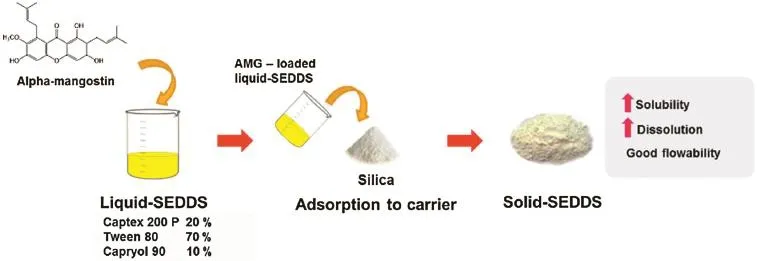
Fig.1–Preparation process of alpha-mangostin solid self-emulsifying drug delivery system.
R E F E R E N C E S
[1]Obolskiy D,Pischel I,Siriwatanametanon N,et al.Garcinia mangostana L.:a phytochemical and pharmacological review. Phytother Res 2009;23:1047–1065.
[2]Qi X,Qin J,Ma N,et al.Solid self-microemulsifying dispersible tablets of celastrol:formulation development,charaterization and bioavailability evaluation.Int J Pharm 2014;472(1–2):40–47.
*E-mail address:satit.put@mahidol.ac.th.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.024
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
