Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
Chutim Mnmuti Bnch ChusuwnIsriy TechtnwtBusrt Krchot Porrnee Purnjoti
aThe Government Pharmaceutical Organization,75/1 Rama VI Road,Ratchathewi,Bangkok 10400,Thailand
bInternational Bio Service Co.,Ltd.,999 Golden Jubilee Medical Center,Mahidol University,Salaya,Nakhon Pathom 73170,Thailand
Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
Ekawan Yoosakula,*,Jaturavit Vattanarongkupa,Chutima Manamutia, Bancha Chuasuwana,Isariya Techatanawata,Busarat Karachota, Porranee Puranajotib
aThe Government Pharmaceutical Organization,75/1 Rama VI Road,Ratchathewi,Bangkok 10400,Thailand
bInternational Bio Service Co.,Ltd.,999 Golden Jubilee Medical Center,Mahidol University,Salaya,Nakhon Pathom 73170,Thailand
A R T I C L E I N F O
Article history:
Available online 25 November 2015
Abacavir
Lamivudine
Bioequivalence
LC-MS/MS
Pharmacokinetics
Abacavir/lamivudine is a combination of two synthetic nucleoside analogues which is indicated in antiretroviral combination therapy for the treatment of human immunodefciency virus (HIV)infection in adults and adolescents[1].A generic product of abacavir/lamivudine has been developed with lower price by the Government Pharmaceutical Organization(GPO)to be an alternative choice of related physicians and patients who will gain access to the lower price medicines at the same quality and safety as the reference product.A comparative randomized,single dose,two-way crossover,open-label bioequivalence study of a generic abacavir/lamivudine(600/300-mg)tablets, alacovir of GPO,Thailand and the reference product,Kivexa of Glaxo Operation UK,United Kingdom in healthy Thai volunteers under fasting conditions was conducted with 7 days washout period between the treatments to compare the rate and extent of absorption and evaluate the safety of the formulations of abacavir and lamivudine.Blood samples were collected at predefned time points up to 24 hours.Plasma samples of subjects were analyzed for abacavir and lamivudine using a validated LC-MS/MS method.Non-compartmental model was used for pharmacokinetic analysis and statistical analysis for twenty-seven subjects who completed both treatments.The 90%parametric confdence intervals for the ln-transformed test/ reference ratios of AUC0-tlast,AUC0-∞and Cmaxwere 101.9(98.37–105.47),101.8(98.42–105.37)and 106.2(98.53–114.54),respectively for abacavir and 102.9(96.24–110.07),102.4(96.08–109.20)and 104.5(96.36–113.37),respectively for lamivudine.These values were within the acceptable range of 80.00–125.00[2].Both formulations were well tolerated.No clinically signifcant or serious ADR was observed.The results of statistical analysis showedthat both formulations were bioequivalent in terms of rate and extent of absorption.Therefore,this study confrmed that both formulations can be used interchangeably.
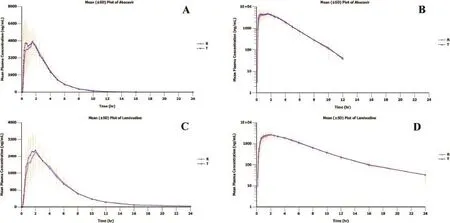
Fig.1–Linear plot of mean(±SD)plasma concentrations-time profles and their semi-logarithmic plot after administration of test product(T)and reference product(R)of abacavir(A,B)and lamivudine(C,D).
Acknowledgements
This study was sponsored by the Government Pharmaceutical Organization,Thailand.The authors would like to thank Ms. Wilak Vangkanonta and Ms.Valaimon Vimolprasan for their assistance.
R E F E R E N C E S
[1]ViiV Healthcare.Prescribing information of Kivexa(abacavir/ lamivudine)600/300 mg tablets,Revised August,2014.
[2]EMA.Guideline on the investigation of bioequivalence. Committee for Medicinal Products for Human Use,EMEA,
*E-mail address:y.ekawan@gpo.or.th.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.019
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
- Factors affecting formation of nanoemulsions containing modifed coconut oil and spearmint oil
