Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
,d
aDepartment of Veterinary Bioscience and Veterinary Public Health,Faculty of Veterinary Medicine,Chiang Mai University,Chiang Mai 50100,Thailand
bNanoscience and Nanotechnology Program,The Graduate School,Chiang Mai University,Chiang Mai 50200, Thailand
cDepartment of Food Animal Clinic,Faculty of Veterinary Medicine,Chiang Mai University,Chiang Mai 50100, Thailand
dDepartment of Pharmaceutical Sciences,Faculty of Pharmacy,Chiang Mai University,Chiang Mai 50200, Thailand
Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
Raktham Mektrirata,*,Kantaporn Janngeonb,Surachai Pikulkaewc, Siriporn Okonogib,d
aDepartment of Veterinary Bioscience and Veterinary Public Health,Faculty of Veterinary Medicine,Chiang Mai University,Chiang Mai 50100,Thailand
bNanoscience and Nanotechnology Program,The Graduate School,Chiang Mai University,Chiang Mai 50200, Thailand
cDepartment of Food Animal Clinic,Faculty of Veterinary Medicine,Chiang Mai University,Chiang Mai 50100, Thailand
dDepartment of Pharmaceutical Sciences,Faculty of Pharmacy,Chiang Mai University,Chiang Mai 50200, Thailand
A R T I C L E I N F O
Article history:
Available online 25 November 2015
Clove
Essential oil
Microemulsion
Cytotoxicity
Infammation
Clove oil is the essential oil of Syzygium aromaticum Merr.and L.M.It is widely used in pharmaceutical applications because of its biological potential including anesthetic,analgesic,antiinfammatory,antibacterial and antioxidant properties[1].The pharmacological effcacy is hindered by the high hydrophobicity of the essential oil;therefore,a thermodynamically stable microemulsion is an alternative attractive preparation for overcoming this problem[2].However,high surfactant concentration used in microemulsion may cause toxicity and other disadvantage to the formulation.This study aimed to investigate the immunotoxic effects of clove oil microemulsion in mice.
The essential oil was isolated from clove bud using simultaneous steam-distillation.Chemical characterization was analyzed using gas chromatograph coupled to a mass spectrometer(GC–MS).The result showed that oil was composed of eugenol(96.11%),caryophyllene(1.34%)and naphthalene (0.63%).The amount of eugenol is related to that previously reported[3].Clove oil microemulsion was formulated from the ternary phase diagram,constructed by using clove oil 10%w/ w,Tween20 and distilled water(Fig.1).Cytotoxic effect of the microemulsion was tested on murine peritoneal macrophages by XTT reduction assay.Infammatory effect was evaluated by intraperitoneal injection in laboratory mice.The result demonstrated mild cytotoxic effect of clove oil microemulsion on peritoneal macrophages(21.71±4.03%).It was found that the cytotoxicity was affected by vehicle substance where clove oilshowed no cytotoxic effect(p>0.05).The current study shows that high surfactant concentration causes in vitro cytotoxicity[4].However,it does not play a role in the infammatory response in mice.Our results contribute to understanding the cytotoxicity and infammatory ability of clove oil microemulsion which has a predictive value with regard to its safety. However,proper composition in pharmaceutical preparation and the pharmacological application needs to be further investigated.
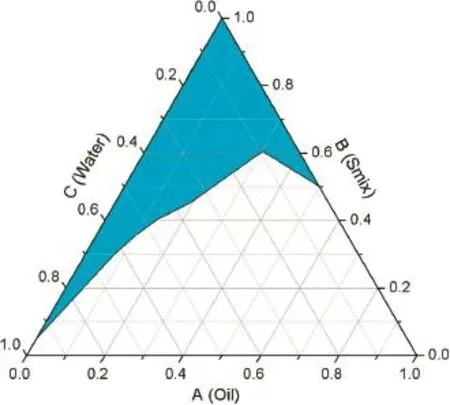
Fig.1–Ternary phase diagram of clove oil,Tween 20 and water.
Acknowledgements
This work was supported by research budget of the National Research Council ofThailand(R000012795)and the Royal Golden Jubilee granted by Thailand Research Fund(5.NS.CM/56/A.1). We also thank Faculty of Veterinary Medicine and Faculty of Pharmacy,Chiang Mai University for providing laboratory facilities.
R E F E R E N C E S
[1]Shaaban HAE,El-Ghorab AH,Shibamoto T.Bioactivity of essential oils and their volatile aroma components:review. J Essent Oil Res 2012;24:203–212.
[2]Gupta S,Moulik SP.Biocompatible microemulsions and their prospective uses in drug delivery.J Pharm Sci 2008;97(1):22–45.
[3]Guan W,Shufen L,Ruixiang Y,et al.Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and three other traditional extraction methods.Food Chem 2007;101:1558–1564.
[4]Arechabala B,Coiffard C,Rivalland P,et al.Comparison of cytotoxicity of various surfactants tested on normal human fbroblast cultures using the neutral red test,MTT assay and LDH release.J Appl Toxicol 1999;19(3):163–165.
*E-mail address:raktham.m@cmu.ac.th.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.020
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
- Factors affecting formation of nanoemulsions containing modifed coconut oil and spearmint oil
