A study on the plasticization of sustained release coatings for their ability to withstand the damaging effects of compaction on coated pellets
,Sivrm Nllmolu,*,Pul Wn Si Heng
aDepartment of Pharmaceutical Technology,International Medical University,57000 Malaysia
bDepartment of Pharmacy,National University of Singapore,117543 Singapore
A study on the plasticization of sustained release coatings for their ability to withstand the damaging effects of compaction on coated pellets
Chan Pui Sheona,Sivaram Nallamolua,*,Paul Wan Sia Hengb
aDepartment of Pharmaceutical Technology,International Medical University,57000 Malaysia
bDepartment of Pharmacy,National University of Singapore,117543 Singapore
A R T I C L E I N F O
Article history:
Available online 24 November 2015
MUPS
Tableting
Plasticizer(TEC)
Pellets
Pharmaceutical solid dosage forms are commonly coated to modify the release of drugs.Due to the disadvantages of coated single-unit dosage forms,such as occurrences of dose dumping and local irritation,coated multi-particulates are preferred. Coated multi-particulates can eventually be flled into capsules or compressed into tablets.The latter is more desirable as unit production costs of tablets are considerably lower and machinery is more easily available.However,compression forces can result in structural changes to the coat,affecting its function.Hence,it is important to understand the factors affecting coat damage during compression[1–3].
The objective of this project is to examine how plasticization of coatings mitigate the level of pellet coat damage in tableted multi-unit pellet system(MUPS).The method involves coating of sustained release coats of different levels of plasticizertriethylcitrate(TEC)byflmcastingmethod.Thecompacts were made with the coated pellets.The casted flms were studied for their mechanical properties and the coated pellets for their dissolution study.The model drug chlorpheniramine maleate was loaded onto sugar coated pellets of 500–600 μm in size using HPMC,povidone and de-ionized water.The drug loaded pellets were flm coated with aqueous ethyl cellulose dispersion with varying levels of TEC using precision coating unit.The coated pellets were formulated with lactose, crospovidone and magnesium stearate into compact masses, by using 15 mm fat face punches with a compression force of 10 kN.These compacted tablets of MUPS were studied for invitro tests like hardness,disintegration and dissolution rate. The surface morphology of the pellets was assessed by using SEM and the results were statistically analyzed by ANOVA.
The results of hardness,disintegration and dissolution were shown in Fig.1.When the amount ofTEC increased,the tablet hardness decreased which comparably produced soft and easily breakable tablets.The disintegration test shows increase in disintegration time with increasedTEC concentration.A signifcant sustain action was observed in 2%weight gain,rather than 10% weight gain pellets.This was found to be the same in all the varying concentrations of TEC(10%,20%and 30%)signifyingthat there was coat damage in 10%weight gain pellets.It can be concluded that the coated pellets could be compressed into tablets without mechanical damage to the coating membrane when additives of 2%weight gain at 10%TEC was used.
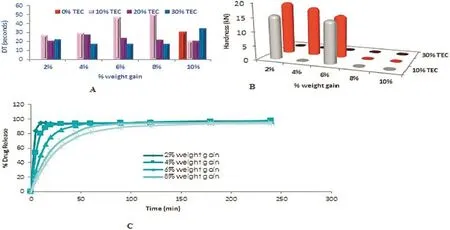
Fig.1–Disintegration Time(A);Hardness(B);Dissolution profle(C)of chlorpheniramine maleate MUPS tablets with varying levels of triethyl citrate plasticizer(TEC).
R E F E R E N C E S
[1]Mizumoto T,Tamura T,Kawai H,et al.Formulation design of an oral,fast-disintegrating dosage form containing taste-masked particles of famotidine.Chem Pharm Bull 2008;56:946–950.
[2]Yao T,Yamadam M,Yamahara H,et al.Tableting of coated particles.II.Infuence of particle size of pharmaceutical additives on protection of coating membrane from mechanical damage during compression process.Chem Pharm Bull 1998;46: 826–830.
[3]Ong LW,Ong KT,Heng PWS.Novel flm modifers to alter the physical properties of ethyl cellulose flms.Pharm Res 2005;22:476–489.
*E-mail address:sivaram_nallamolu@imu.edu.my.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.032
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
