Novel approaches for posterior segment ocular drug delivery with folate-modifed liposomal formulation
Toshiki Hayashi,Risako Onodera,Kohei Tahara,Hirofumi Takeuchi
Gifu Pharmaceutical University,1-25-4 Daigaku-nishi,Gifu 501-1196,Japan
Novel approaches for posterior segment ocular drug delivery with folate-modifed liposomal formulation
Toshiki Hayashi*,Risako Onodera,Kohei Tahara,Hirofumi Takeuchi
Gifu Pharmaceutical University,1-25-4 Daigaku-nishi,Gifu 501-1196,Japan
A R T I C L E I N F O
Article history:
Available online 25 November 2015
Liposome
Retina
Eyedrop
Active targeting
The leading causes of vision impairment and blindness are posterior segment-related diseases including age-related macular degeneration,diabetic macular edema and endophthalmitis. Recently,pharmaceutical approaches to these diseases have used steroids and oligonucleotides.These drugs are usually administered via invasive injection because noninvasive delivery method such as eye drop administration of drugs is not available.However,repeated injections are associated with potential risks of complications.Moreover,patients may not comply with such regimens.Thus,there is a pressing need for noninvasive delivery systems targeting the posterior segment of the eye.In previous studies,we have reported the potential of liposomal eye drop formulation composed of submicron-sized liposomes(ssLip)as a novel system for delivering drugs to the posterior segment of the eye,including the retina[1,2].In a series of our studies using ssLips containing a fuorescence marker,coumarin-6,the die was shown to be delivered into the retina after eyedrop administration in mice,rabbits,and monkeys[2].The purpose of this study was to improve the drug delivery effciency to the retina by folate-modifcation of liposomes.We designed folate-modifed ssLip(FA-modifed ssLip) to aim at selective targeting of the folic acid receptor,which is expressed on retinal pigment epithelia cells.This active targeting may lead to increase in the delivery effciency of the liposomal systems.
The ssLip containing coumarin-6 was prepared using hydration method,and the folate ligand was inserted into preformed liposomes by the post insertion technique[3].A single dose of 3 μL of the liposomal formulation was dropped onto the left eye of mice.The mice were then sacrifced 15,30, 60,120,180,240 min after the administration of the liposomal formulation.Frozen section of the enucleated eyes was prepared with a cryostat.Images were obtained using an epifuorescence microscope and were analyzed using Image J software.Fig.1 shows the typical epifuorescence microscopic images of the retina 0,30,120 min after eyedrop administration.To estimate the delivery effciency to the retina, the magnitude of fuorescence emission in the innerplexiform layer(IPL)of the retina was quantifed using Image J software.The fuorescence intensity of unmodifed ssLip showed the maximal value at 15–30 min,thereafter pronounced decrease was observed up to 120 min.On the other hand,FA-modifed ssLip showed the higher intensity whole range of the period after the administration compared to that of unmodifed ssLip.Besides,the liposomes tested in ocular cells showed little cytotoxicity.The FA-modifcation of liposomes may advance the delivery effciency of compounds to the retina.
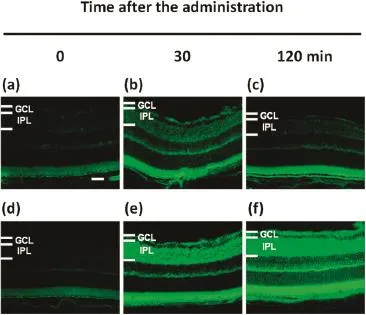
Fig.1–Epifuorescence microscopic images of the retina after defnite intervals of eyedrop administration.(a–c) Unmodifed ssLip,(d–f)FA-modifed ssLip.
R E F E R E N C E S
[1]Hironaka K,Inokuchi Y,Tozuka Y,et al.Design and evaluation of a liposomal delivery system targeting the posterior segment of the eye.J Control Release 2009;136:247–253.
[2]Inokuchi Y,Hironaka K,Fujisawa T,et al.Physicochemical Properties affecting retinal drug/coumarin-6 delivery from nanocarrier systems via eyedrop administration.Invest Ophthalmol Vis Sci 2010;51:3162–3170.
[3]Takeuchi H,Yamamoto H,Niwa T,et al.Mucoadhesion of polymer-coated liposomes to rat intestine in vitro.Chem Pharm Bull 1994;42:1954–1956.
*E-mail:106032@gifu-pu.ac.jp
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.036
1818-0876/©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
