Effect of cationic microemulsion on hemolysis and immune cell uptake
Vimolms Lipipun,Grnpimol C.Ritthidej
aDepartment of Pharmaceutics and Industrial Pharmacy,Chulalongkorn University,Bangkok 10330,Thailand
bDepartment of Biochemistry and Microbiology,Faculty of Pharmaceutical Sciences,Chulalongkorn University, Bangkok 10330,Thailand
Effect of cationic microemulsion on hemolysis and immune cell uptake
Sakalanunt Lamaisakula,*,Angkana Tantituvanonta, Vimolmas Lipipunb,Garnpimol C.Ritthideja
aDepartment of Pharmaceutics and Industrial Pharmacy,Chulalongkorn University,Bangkok 10330,Thailand
bDepartment of Biochemistry and Microbiology,Faculty of Pharmaceutical Sciences,Chulalongkorn University, Bangkok 10330,Thailand
A R T I C L E I N F O
Article history:
Available online 25 November 2015
Cationic microemulsion
Hemolysis
Adjuvant
Immune cell uptake
Squalene-based oil-in-water nanoemulsions,constituents in infuenza vaccines,have been approved in Europe as human licensed adjuvants[1].With similar constituents but easier manufacturing procedure and greater physical stability[2], microemulsion was of interest.To improve cellular uptake, positive surface charge of nanosystems has been reported [3].In the present study,cationic microemulsions were prepared,characterized and evaluated in immune cell,human monocyte/macrophage.Toxicity of formulations was also investigated.
Oil in water microemulsions containing vegetable oil and nonionic surfactants were prepared in the absence and presence of cationic surfactant,cetyltrimethylammonium Bromide (CTAB),designated as C0,C2 and C4,respectively and then sterilized by autoclaving.Prior to evaluation,each formulation was diluted with suspension of BSA,a model antigen,by simple mixing.The results from zetasizer Nano-ZS indicated that the average particle size of the autoclaved preparations was about 20 nm with pdI between 0.20 and 0.33 and was stable over 18 months at 4°C in the refrigerator.Addition of cationic surfactant gradually increased the zeta potential value of microemulsion.Furthermore,BSA in all formulations had retained protein integrity of both primary and secondary structures,assessed by SDS-PAGE method and circular dichroism spectroscopy,respectively.Hemolytic activity of the formulations when incubated with a suspension of RBC was not more than 0.1%equivalent to that of a negative saline control,indicating good hemocompatibility as shown in Fig.1A. Cellular uptake results from FACS fow cytometry analysis on human monocyte/macrophage cells between prepared BSA formulations and commercial AddaVax™ adjuvant,shown in Fig.1B,revealed that the obtained formulations with higher amount of cationic surfactant tended to signifcantly improve cellular uptake of the blended FITC-BSA(p<0.05). These results were confrmed by confocal microscopy images of internalization of FITC-BSA by elicited human monocyte/macrophage.According to these results,it was concluded that the prepared cationic microemulsion hadpotential to be used as novel adjuvant for parenteral vaccine administration.
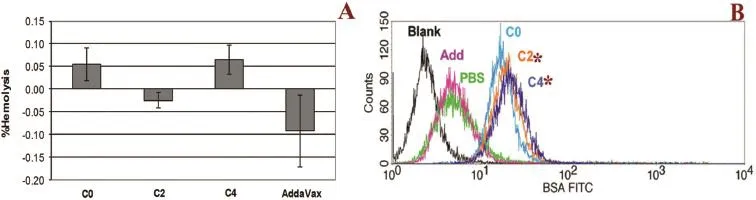
Fig.1–Hemolysis assay(A);overlay histograms of fow cytometry analysis of cellular uptake study(B).
Acknowledgements
Research grant from the Offce of Higher Education Commission via Chulalongkorn University,fscal year 2014–2015 is acknowledged.Appreciation is to Noppadol Sa-Ard-Iam(Unit Cell of Immunopathological/Clinical Research in Periodontal Disease,Faculty of Dentistry,Chulalongkorn University)for FACS fow cytometry study.
R E F E R E N C E S
[1]Coffman RL,Sher A,Seder RA.Vaccine adjuvants:putting innate immunity to work.Immunity 2010;33(4):492–503.
[2]Jain J,Fernandes C,Patravale V.Formulation development of parenteral phospholipid-based microemulsion of etoposide. AAPS Pharm Sci Tech 2010;11(2):826–831.
[3]Ahsan F,Rivas IP,Khan MA,et al.Targeting to macrophages: role of physicochemical properties of particulate carriers–liposomes and microspheres–on the phagocytosis by macrophages.J Control Release 2002;79(1–3):29–40.
*E-mail address:sakalanunt.L@student.chula.ac.th.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.066
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
