Development of triple-layered tablets containing laxatives with a hydrophilic polymer
GL PharmTech Corporation,#714,Jungang Induspia V,137,Sagimakgol-ro,Jungwon-gu,Seongnam,Gyeonggido,Republic of Korea
Development of triple-layered tablets containing laxatives with a hydrophilic polymer
Woo Heon Song,Jin Seob Oh,Kyung Soo Lee,Jun Sang Park*
GL PharmTech Corporation,#714,Jungang Induspia V,137,Sagimakgol-ro,Jungwon-gu,Seongnam,Gyeonggido,Republic of Korea
A R T I C L E I N F O
Article history:
Available online 25 November 2015
Hydrophilic polymer
Sodium carboxymethylcellulose
Bisacodyl
Triple-layered tablet
Constipation is a common functional gastrointestinal disorder which has caused much discomfort affecting the quality of life.The prevalence of constipation in the general population is approximately 20%[1].Various kinds of laxatives were introduced such as bulking agent,stool softener,stimulant,and osmotic agent[2].Fixed dose combination with bisacodyl as a stimulant and docusate sodium as a stool softener,Ducolax STM,was developed as enteric coated tablets targeting colon. Hydrophilic polymers have the ability to absorb a large amount of water in the gastrointestinal tract,which has been reported to be useful to treat constipation[3].We focus on the possible synergic effect of hydrophilic polymer with bisacodyl. The aim of this study was to develop enteric coated triplelayered tablet formulation containing bisacodyl and docusate sodium with hydrophilic polymer and investigate the synergistic effect by loperamide-induced constipation model in rat.
Sodium carboxymethylcellulose was selected among hydrophilic polymers.Bi-layered and triple-layered tablet formulations were prepared because hydrophilic polymer negatively affects the dissolution profles of bisacodyl and docusate sodium.Tablets were enteric coated using an aqueous dispersion of Acryl-Eze IITMto ensure the gastric resistance and drug delivery to the colon region.In vitro drug dissolution test was conducted according to USP Apparatus 2 at 100 rpm in pH 7.5 phosphate buffer after an acid stage in 0.1 N hydrochloride for 2 hours and analyzed the released drug using reversed phase HPLC at a wavelength of 210 nm.In loperamide-induced constipation model rat,the synergistic effect of sodium carboxymethylcellulose with bisacodyl was evaluated by checking the change of stool weight.The bi-and triple-layered tablets were successfully manufactured without any troubles during the tableting process.The bi-layered tablets induced the critical problem related to stability because bisacodyl is easily hydrolyzed upon contact with sodium carboxymethylcellulose,while triple-layered tablets with inactive middle layer drastically improved the stability.Enteric coating with an aqueous dispersion of AcrylEze IITMshowed acceptable acid resistance in 0.1 N HCl and pH 4.5 acetate buffer for 2 hours, although some of the marketed products failed in pH 4.5.Triplelayered prototype indicated comparable acid resistance anddissolution profle with Ducolax STM(Fig.1).Sodium carboxymethylcellulose co-administrated with bisacodyl increased the weight of stools in loperamide-induced constipation model rats in comparison with bisacodyl administrated group showing the synergistic effect.
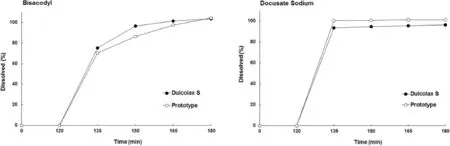
Fig.1–Comparative dissolution profles of prototype and Ducolax STM.
Acknowledgements
The authors acknowledge the fnancial support for this work received from the Korean Small and Medium Business Administration(Project No.S2223760).
R E F E R E N C E S
[1]Higgins PD,Johanson JF.Epidemiology of constipation in North America:a systemic review.Am J Gastroenterol 2004;99(4):750–759.
[2]Emmanuel AV,Tack J,Quigley EM,et al.Pharmacological management of constipation.Neurogastoenterology Motil 2009;21:41–54.
[3]Takaharu S,Fujie M,Yuju I,et al.Laxative and anti-diarrheal activity of polycarbophil in mice and rats.Jpn J Pharmacol 2002;89:133–141.
*E-mail address:jspark@glpt.co.kr.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.090
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
