Film coated foating tablets using sublimable substances
,Stit Puttipiptkhchorn, Pornsk Srimornsk,Srisgul Sungthongjeen,
aDepartment of Pharmaceutical Technology,Faculty of Pharmaceutical Sciences,NaresuanUniversity, Phitsanulok 65000,Thailand
bDepartment of Manufacturing Pharmacy,Faculty of Pharmacy,Mahidol University,Bangkok 10400,Thailand
cDepartment of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
dPharmaceutical Biopolymer Group(PBiG),Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
Film coated foating tablets using sublimable substances
Worawut Kriangkraia,Satit Puttipipatkhachornb, Pornsak Sriamornsakc,d,Srisagul Sungthongjeena,*
aDepartment of Pharmaceutical Technology,Faculty of Pharmaceutical Sciences,NaresuanUniversity, Phitsanulok 65000,Thailand
bDepartment of Manufacturing Pharmacy,Faculty of Pharmacy,Mahidol University,Bangkok 10400,Thailand
cDepartment of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
dPharmaceutical Biopolymer Group(PBiG),Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
A R T I C L E I N F O
Article history:
Available online 24 November 2015
Floating tablets
Coating
Sublimable substance
Floating properties
Drug release
During the past few decades foating drug delivery systems (FDDSs)have been developed to prolong gastric retention time and obtain suffcient drug bioavailability[1].To avoid unpredictable time to foat due to variable pH of the gastric fuid in each subject and food in the stomach[2],sublimation technique is the new interesting approach to prepare noneffervescent FDDSs[3].The objective of the present study was to develop the low-density flm coated foating tablets using sublimable substances.
To prepare low density porous core tablets,model drug(anhydrous theophylline),fller(e.g.,microcrystalline cellulose, HPMC),and sublimable substance(ammonium carbonate)were mixed for 10 min prior to addition of magnesium stearate(0.5% w/w)and Aerosil®200(0.5%w/w).The powder mixture was further mixed for 3 min and compressed into tablets(diameter,9.53 mm;biconvex;hardness,9–10 kg;average tablet weight,300 mg)using a single punch tableting machine(Model YH06,Yeo Heng Co.,Ltd.,Thailand).The tablets were incubated at 70°C for 72 h to eliminate sublimable substance.The porous tablets were coated with Eudragit®RL 30D plasticized with 20%w/w diethyl phthalate.Coating condition is as follows:batch size,1 kg;preheating temperature,50°C;preheating time,30 min;inlet temperature,48–50°C;outlet temperature,39–41°C;atomizing air pressure,2.5 bar;spray rate,5–8 mL/min.
The results exhibited that addition and increasing level of sublimable substance increased porosity of core tablet,leading to reduction of density and hardness of the tablet.Although the porous core tablets could foat immediately,the foating time of the core was quite short.Therefore,the polymericmembrane was needed.The SEM photomicrograph of a crosssection of flm coated foating tablets demonstrates the porous structure of the core tablet coated with Eudragit®RL30D flm (Fig.1).The flm coated foating tablets using sublimable substance could initially foat due to low-density of the systems (<1 g/cm3)and maintain foatation more than 8 h with sustained drug release as required in this study.Eudragit®RL30D coating might not only plays a role to control the drug release, but also entrap air in the porous structure of the core tablet and maintain low density of the system.
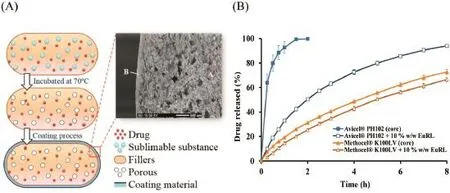
Fig.1–Schematic and SEM photomicrograph of a cross-section of flm coated foating tablets(40%w/w ammonium carbonate,10%w/w Eudragit®RL30D)(A)and drug release profles(B).
Acknowledgments
This work was fnancially supported by the Royal Golden Jubilee Ph.D.Program(RGJ)(Grant No.PHD/0340/2551)under the Thailand Research Fund(TRF),Thailand and the Embassy of France in Thailand.
R E F E R E N C E S
[1]Sungthongjeen S,Sriamornsak P,Puttipipatkhachorn S. Design and evaluation of foating multi-layer coated tablets based on gas formation.Eur J Pharm Biopharm 2008;69:255–263.
[2]Fukuda M,Peppas NA,McGinity JW.Floating hot-melt extruded tablets for gastroretentive controlled drug release system.J Control Release 2006;115:121–129.
[3]Oh TO,Kim JY,Ha JM,et al.Preparation of highly porous gastroretentive metformin tablets using a sublimation method.Eur J Pharm Biopharm 2013;83:460–467.
*E-mail address:sungts2000@yahoo.com.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.093
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
