Preparation and characterization of solidifed SMEDDS containing furbiprofen by spray drying method
Yoo-Jeong Jang,Kwan Hyung Cho
College of Pharmacy,Inje University,197 Inje-ro,Gimhae 621-749,Republic of Korea
Preparation and characterization of solidifed SMEDDS containing furbiprofen by spray drying method
Yoo-Jeong Jang,Kwan Hyung Cho*
College of Pharmacy,Inje University,197 Inje-ro,Gimhae 621-749,Republic of Korea
A R T I C L E I N F O
Article history:
Available online 25 November 2015
Flurbiprofen
Solidifed SMEDDS
Solubilization
Flurbiprofen,a non-steroidal anti-infammatory agent,is used to treat rheumatoid arthritis and sore throat[1].However,it gave poor water solubility,and various solubilization technique such as self-microemulsifying drug delivery system (SMEDDS)has been used to improve the solubility,dissolution and oral bioavailability[2].
The objective of this work was to develop redispersible solidifed SMEDDS containing water-insoluble furbiprofen with enhanced solubility.Various compositions were investigated with medium chain triglyceride,surfactant and co-surfactant, to obtain stable SMEDDS.The selected SMEDDS was dissolved in 70%ethanol and adsorbed to fumed silicon dioxide with the weight ratio of 1/1 by spray drying method.The powder characteristics of the solidifed SMEDDS were determined by DSC and microscopic observation.The solidifed SMEDDS was dispersed in water and the particle size distribution was compared to the initial SMEDDS.The SMEDDS composed of Cremophor EL and Capryol 90 with 1/1 weight ratio without co-surfactant,dissolved furbiprofen of 20 mg/mL and showed transparent dispersion in water.The solidifed SMEDDS appeared to be small uniform particles in the microscopic observation with fowing property.In the DSC,the peak melting of crystalline furbiprofen disappeared in the solidifed SMEDDS, which mean the non-crystalline state of furbiprofen.The mean particle size of the redispersed solidifed SMEDDS(273.5 nm) in water was about 13-fold higher than the initial SMEDDS (20.1 nm).However,the solidifed SMEDDS gave the physically stable SMEDDS without phase separation during a week. In conclusion,it would mean that the solidifed SMEDDS formed emulsion spontaneously in water and improved the solubility of water-insoluble furbiprofen.
Acknowledgements
The authors wish to thank Inje University,Gimhae,South Korea, for research funding.
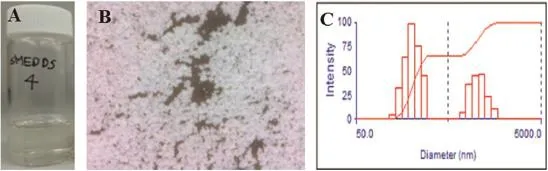
Fig.1–SMEDDS photograph(A),solidifed SMEDDS photograph(B)and particle size distribution of solidifed SMEDDS(C).
R E F E R E N C E S
[1]Davies NM.Clinical pharmacokinetics of furbiprofen and its enantiomers.Clin Pharmacokinet 1995;28:100–114.
[2]Balakrishnan P,Lee BJ,Oh DH,et al.Enhanced oral bioavailability of dexibuprofen by a novel solid selfnanoemulsifying drug delivery system(SEDDS).Eur J Pharm Biopharm 2009;72:539–545.
*E-mail address:chokh@inje.ac.kr.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.103
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
