Swelling and deswelling kinetics of polyvinyl alcohol and lactic acid hydrogel flms
, Jirpornchi Sukseree,c
aThe Herbal Medicinal Products Research and Development Center(Cooperation between Rangsit University, Harbin Institute of Technology,and Heilongjiang University of Chinese Medicine),Faculty of Pharmacy,Rangsit University,Muang,Pathum Thani 12000,Thailand
bSun Herb Thai Chinese Manufacturing Plant,Faculty of Pharmacy,Rangsit University,Muang,Pathum Thani 12000,Thailand
cDepartment of Pharmaceutical Chemistry,Faculty of Pharmacy,Rangsit University,Muang,Pathum Thani 12000,Thailand
Swelling and deswelling kinetics of polyvinyl alcohol and lactic acid hydrogel flms
Natawat Chankanaa,b,*,Chitradee Luprasongb,Chaowalit Montona, Jirapornchai Suksaereea,c
aThe Herbal Medicinal Products Research and Development Center(Cooperation between Rangsit University, Harbin Institute of Technology,and Heilongjiang University of Chinese Medicine),Faculty of Pharmacy,Rangsit University,Muang,Pathum Thani 12000,Thailand
bSun Herb Thai Chinese Manufacturing Plant,Faculty of Pharmacy,Rangsit University,Muang,Pathum Thani 12000,Thailand
cDepartment of Pharmaceutical Chemistry,Faculty of Pharmacy,Rangsit University,Muang,Pathum Thani 12000,Thailand
A R T I C L E I N F O
Article history:
Available online 24 November 2015
Hydrogel flms
Swelling
Deswelling
In this work,we studied the swelling and deswelling kinetics of polyvinyl alcohol(PVA)and lactic acid(LA)hydrogel flms between distilled water and acetone phase.The hydrogel flms were prepared by dissolve 5.0 g of PVA in hot water.Then,7.5 g LA solution was slowly added into the clearly PVA solution at room temperature.After that,the mixture solution was subsequently cured in an oven for different curing times at 100°C. The swelling and deswelling kinetics of hydrogel flms were determined by immerse the hydrogel flms in distilled water and acetone at room temperature.The hydrogel flms were weighted at different time(Wt).The swelling and deswelling were calculated in terms of the relative hydrogel volume(Vrel) using this equation whenW was its equilibrated swollen weight of hydrogel flms in distilled water[1].
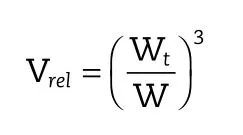
Fig.1 shows the swelling and deswelling kinetics of hydrogel flms,the initial swollen hydrogel flms volume began at 1 value of Vrel.They could be recovered after collapsing the hydrogel flms in acetone within 20–30 min(Vrel=0). However,all hydrogel flms quickly swell within 50 min,but they could not attain to its initial volume(Vrel=1)after transferring to distilled water again.On the second cycle, this process required much shorter times(not more than 20 min)than those of the corresponding hydrogel flms.The surface of hydrogel flms would be initially large by start to relax formation after immersed in distilled water,water absorption of the hydrogel flms occured easily.However,the mixing time and curing time not signifcantly affected on swelling and deswelling process.The swelling and deswelling kinetics were very important.They would be used to prepare the pharmaceutical formulation and controlled drug release.
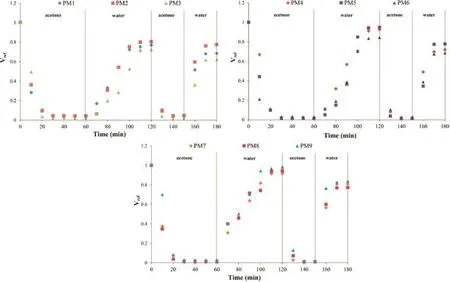
Fig.1–Swelling and deswelling kinetics of hydrogel flms(PM1,2,3 mixed for 30 min,PM4,5,6 mixed time for 60 min,and PM7,8,9 mixed for 90 min with curing time for 120,150,and 180 min,respectively at 100°C).
Acknowledgements
The authors acknowledge the fnancial support from the Research Institute of Rangsit University(Grant no. 36/2557).
R E F E R E N C E
[1]Ceylan D,Ozmen MM,Okay O.Swelling–deswelling kinetics of ionic poly(acrylamide)hydrogels and cryogels.J Appl Polym Sci 2006;99(1):319–325.
*E-mail address:natawat.c@rsu.ac.th.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.104
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
