Chemical analysis of hydroquinone and retinoic acid contents in facial whitening creams
aHealth Promotion and Diseases Prevention Department,Health and Diseases Control Unit,Nay Pyi Taw, Myanmar
bPharmaceutical Chemistry Department,Myanmar
cPharmaceutics Department,University of Pharmacy,Yangon,Myanmar
dDepartment of Pharmacy,Military Institute of Nursing and Paramedical Sciences,Yangon,Myanmar
Chemical analysis of hydroquinone and retinoic acid contents in facial whitening creams
Aung Myo Hteta,*,Ei Ei Thinb,Mi Mi Sawc,Soe Wind
aHealth Promotion and Diseases Prevention Department,Health and Diseases Control Unit,Nay Pyi Taw, Myanmar
bPharmaceutical Chemistry Department,Myanmar
cPharmaceutics Department,University of Pharmacy,Yangon,Myanmar
dDepartment of Pharmacy,Military Institute of Nursing and Paramedical Sciences,Yangon,Myanmar
A R T I C L E I N F O
Article history:
Available online 25 November 2015
Hydroquinone and retinoic acid HPLC
Facial whitening creams
Hydroquinone is potentially carcinogenic and is known to be a skin and respiratory irritant.Hydroquinone is carcinogenic it has been banned in some countries because of fears of a cancer risk[1,2].Animal studies have shown that topically applied retinoic acid can be photocarcinogenic[3].
The aim of this study was qualitative and quantitative determination of hydroquinone and retinoic acid contents in facial whitening creams by colour reaction tests,FT-IR after TLC separation and HPLC.The HPLC optimized chromatographic condition was methanol:water:acetic acid(95:5:0.15, v/v/v),pH 5.2,isocratic elution with fow rate of 0.5 mL min−1, injection volume 10 μL and UV detection wavelength at 280 nm. The developed HPLC method gave a good sensitivity(LOD and LOQ were 0.03 μg mL−1and 0.09 μg mL−1for hydroquinone and 0.06 μg mL−1and 0.18 μg mL−1for retinoic acid).The calibration curves were linear(r2>0.9995)over hydroquinone and retinoic acid concentration range of 1–9 μg mL−1.The relative standard deviations of the method were 0.36%for injection precision of both analytes,less than 1.7%for intraday precision and less than 1.9%for inter-day precision of hydroquinone and retinoic acid.Validated method was accurate with recoveries in the range of 95.46%to 103.11%for both analytes.
Two samples contained hydroquinone concentrations 2.71% and 8.93%which were higher than the recommended WHO limit of 2%.The detected retinoic acid concentrations in cosmetic samples were 0.0612%and 1.11%.Therefore,this study will be useful to ascertain the safety of cosmetic products for human health.
Acknowledgements
The authors acknowledge Brigadier General Soe Win,Director,Directorate of Medical Service,and Brigadier General Phone Myat,Commandant,Military Institute of Nursing and Paramedical Sciences for their kind permission and encouragement to do this research work.Thanks are also due to Major Myint Myint Shein(retired),Vice president, Myanmar Pharmaceutical Association for the fnancial support.
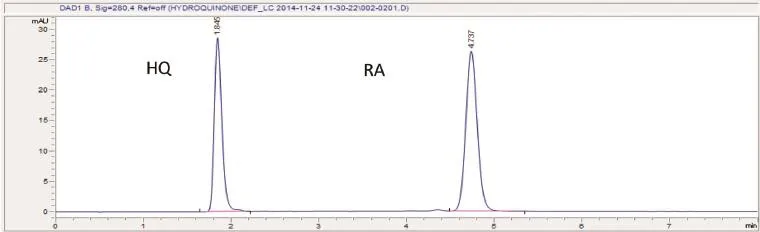
Fig.1–HPLC chromatogram of hydroquinone(HQ)and retinoic acid(RA).
R E F E R E N C E S
[1]Hutson DH,Dean BJ,Brooks TM,et al.Genetic toxicology testing of 41 industrial chemicals.Research 1999;153:57–77.
[2]Engasser P,Maibach H.Cosmetics and dermatology.J Am Acad Dermatol 2003;5:143–147.
[3]Davies RE,Forbes PD.Retinoids and photocarcinogenesis:a review.J.Toxicol Cut Ocular Toxicol 1988;7:241–253.
*E-mail:hydroquinoneamt@gmail.com.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.117
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
