Effect of precipitation/re-dissolution processes from the supersaturated solution on the intestinal absorption of poorly water-soluble drugs
Haruki Higashino,Keiko Minami,Makoto Kataoka,Shinji Yamashita
Faculty of Pharmaceutical Sciences,Setsunan University,Nagao toge-cho 45-1 Hirakata,Osaka 573-0101, Japan
Effect of precipitation/re-dissolution processes from the supersaturated solution on the intestinal absorption of poorly water-soluble drugs
Haruki Higashino*,Keiko Minami,Makoto Kataoka,Shinji Yamashita
Faculty of Pharmaceutical Sciences,Setsunan University,Nagao toge-cho 45-1 Hirakata,Osaka 573-0101, Japan
A R T I C L E I N F O
Article history:
Available online 23 November 2015
Supersaturation
Oral absorption
Precipitation
Re-dissolution
HPMC
As one of the possible technologies to improve the oral absorption of poorly water-soluble drugs,supersaturable formulation,which enables to dissolve the drug to the higher concentration than their equilibrium solubility,is now attracting the attention[1].This include salt-formation,soliddispersion,co-crystallization or the use of amorphous form. Since supersaturation is a thermodynamically metastable state, supersaturated solution has a high potential to precipitate.Some pharmaceutical excipients,such as hydroxypropyl methylcellulose(HPMC),are used as precipitation inhibitors to stabilize the supersaturated state of drugs in the GI tract[2].In addition,re-dissolution process of the precipitated drug might play an important role to improve the oral absorption even though there is little evidence to support it.In this study,for better understanding the in vivo performance of supersaturable formulations,precipitation/re-dissolution processes of BCS class 2 drug from a supersaturated solution were investigated in the presence or absence of HPMC.The contribution of re-dissolution process on the total drug absorption was also evaluated.
Danazol was used as a model drug of BCS class 2 and its supersaturated solution was prepared by solvent shift method [3].Briefy,danazol was completely dissolved in DMSO as a stock solution,then the stock solution was diluted with fasted state simulated intestinal fuid(FaSSIF,pH=6.5)to obtain a supersaturated solution.As a control,danazol was directly dispersed in FaSSIF.Dissolved concentration of the drug was observed in vitro during 4 h,refecting a transit time in human GI tract. As an in vitro index of the dissolved amount of drug,area under the dissolved concentration–time curve(AUCdissolved)was calculated.Further,the precipitation from the supersaturatedsolution was collected to analyze the particle size.In vivo drug absorption was evaluated by intra-intestinal administration experiment in rat.Under the anesthesia,a supersaturated solution was injected into the small intestine,then plasma drug concentration–time profle was observed.
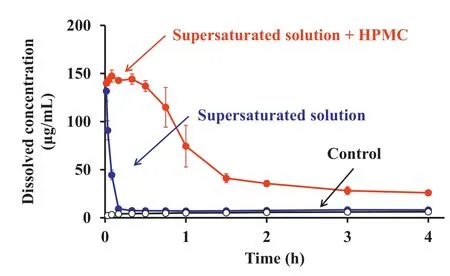
Fig.1–In vitro dissolved concentration-time profle of danazol in FaSSIF.
Dissolved concentration of danazol in the supersaturated solution was about 15%at 1 min after the dilution regardless of the presence/absence of HPMC.Supersaturation disappeared within 30 min in the absence of HPMC,while the addition of HPMC greatly stabilized the supersaturated state, keeping the higher dissolved concentration than that in the control for 4 hours(Fig.1).The ratio of AUCdissolvedto that in the control was 1.7-fold in the absence of HPMC and 11-fold in the presence.However,in the in vivo experiment,addition of HPMC failed to improve the oral absorption of danazol and no signifcant differences were observed in their plasma concentrations.Since danazol is a highly permeable drug,it was considered that the initial dissolved fraction in the supersaturated solution was absorbed quickly from the upper intestine in vivo,then the effect of prolonged supersaturation by HPMC was not observed.Interestingly,the fraction of absorbed from the supersaturated solution was estimated to be 60%,whereas the initial dissolved fraction was only 15%regardless of the presence/absence of HPMC.Since it was found that the particle size of precipitated danazol was much smaller than the original material,it was suggested that the increase in the redissolution rate from the precipitate contributed to the improved absorption of danazol.This study indicated the importance of the re-dissolution process after precipitation from the supersaturated solution for improving the oral absorption of poorly soluble drugs.
R E F E R E N C E S
[1]Brouwers J,Brewster M,Augustijns P.Supersaturating drug delivery systems:the answer to solubility-limited oral bioavailability.J Pharm Sci 2009;98(8):2549–2572.
[2]Ozaki S,Kushida I,Yamashita T,et al.Inhibition of crystal nucleation and growth by water-soluble polymers and its impact on the supersaturation profles of amorphous drugs. J Pharm Sci 2013;102(7):2273–2281.
[3]Bevernage J,Brouwers J,Clarysse S,et al.Drug supersaturation in simulated and human intestinal fuids representing different nutritional states.J Pharm Sci 2010;99(11):4525–4534.
*E-mail address:h-higash@pharm.setsunan.ac.jp.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.10.049
1818-0876/©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
