Design and development of optimal invasomes for transdermal drug delivery using computer program
,c
aDivision of Pharmaceutical Chemistry and Technology,Faculty of Pharmaceutical sciences,Ubon Ratchathani University,Ubon Ratchathani 34190,Thailand
bDepartment of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
cPharmaceutical Development of Green Innovations Group(PDGIG),Faculty of Pharmacy,Silpakorn University, Nakhon Pathom 73000,Thailand
Design and development of optimal invasomes for transdermal drug delivery using computer program
Sureewan Duangjita,c,*,Tassanan Nimcharoenwanb,Nutcha Chomyab, Natthporn Locharoenratb,Tanasait Ngawhirunpatb,c
aDivision of Pharmaceutical Chemistry and Technology,Faculty of Pharmaceutical sciences,Ubon Ratchathani University,Ubon Ratchathani 34190,Thailand
bDepartment of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
cPharmaceutical Development of Green Innovations Group(PDGIG),Faculty of Pharmacy,Silpakorn University, Nakhon Pathom 73000,Thailand
A R T I C L E I N F O
Article history:
Available online 25 November 2015
Design expert
Invasomes
Liposomes
Capsaicin
Capsaicin(CAP)is a major pungent component that has been widely studied in medical and pharmaceutical felds.CAP was used both orally and topically for pain relief.However,the extreme pungency and the water insolubility of CAP lead to its restriction in the development of CAP as drug delivery system [1].Our previous study suggested that the computer software exhibited a benefcial role in the development of menthosomes for transdermal drug delivery[2].To confrm the reliability and reproducibility of simultaneous optimal formulations,the optimal ultrafexible liposomes(invasomes)estimated by the computer software(Design Expert®)were experimentally formulated and investigated.To achieve this purpose,invasomes with Comperlan®KD and d-limonene as potential penetration enhancer were developed.Using a two-factor factorial design with centroid replication as a model experimental design, the invasomes were demonstrated.The model invasome formulations containing a constant composition of 10 mM phosphatidylcholine,1 mM cholesterol and 0.15%capsaicin,and various percentages of d-limonene and Comperlan®KD were prepared.The physicochemical characteristics e.g.,vesicle size, size distribution,zeta potential,entrapment effciency and skin permeability of the model invasome formulations were evaluated.The compositions and the physicochemical characteristics of invasomes were defned as formulation factor(Xn)and response variables(Yn),respectively.The relationship between formulation factor and response variables was predicted,and the optimal invasome formulation was also optimized using Design Expert®.The response surfaces estimated by Design Expert®illustrated obvious relationship between formulation factor and response variables.The formulation factor directlyaffected the physicochemical characteristics of invasomes.The 0.15%capsaicin-loaded invasomes were smaller than 100 nm in size,narrow size distribution(0.01–0.30)and had minor negative zeta potential value(less than−20 mV).The skin permeability of the optimal invasomes was signifcantly higher than conventional liposomes and commercial product(0.15% capsaicin in ethanolic solution).The response surfaces estimated by the computer program were helpful for the development of optimal invasomes for transdermal drug delivery(Fig.1).
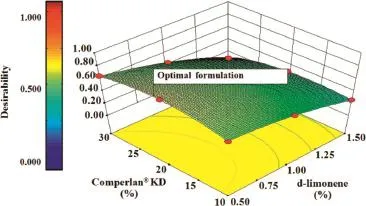
Fig.1–The three dimensional response surface plot of the desirability of invasomes.
Acknowledgments
The authors gratefully acknowledge the Thailand Research Funds through the Basic Research Grant(Grant No.5680016), the Silpakorn University Research and Development Institute(Grant No.SURDI 58/01/10),the Faculty of Pharmaceutical Sciences,Ubon Ratchathani University,and the Faculty of Pharmacy,Silpakorn University,Thailand,for facilities and fnancial support.
R E F E R E N C E S
[1]Duangjit S,Chairat W,Opanasopit P,et al.Application of design expert for the investigation of capsaicin-loaded microemulsions for transdermal delivery.Pharm Dev Technol 2015;21:1–8.
[2]Duangjit S,Obata Y,Sano H,et al.Menthosomes,novel ultradeformable vesicles for transdermal drug delivery: optimization and characterization.Biol Pharm Bull 2012;35(10):1720–1728.
*E-mail address:sureewan.d@ubu.ac.th.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.10.039
1818-0876/©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
