Application of povacoat as dispersion stabilizer of nanocrystal formulation
Shot HoriiHruk Tkeuchi Ktsuy TermotoYuichiro Nkd,Nofumi Hshimoto
aSetsunan University,45-1,Nagaotoge-cho,Hirakata,Osaka 573-0101,Japan
bSanten Pharmaceutical Company Ltd.,40-20,Ofukacho,Kita-ku,Osaka 530-8552,Japan
Application of povacoat as dispersion stabilizer of nanocrystal formulation
Kayo Yuminokia,*,Fuko Sekoa,Shota Horiia,Haruka Takeuchia, Katsuya Teramotoa,Yuichiro Nakadab,Naofumi Hashimotoa
aSetsunan University,45-1,Nagaotoge-cho,Hirakata,Osaka 573-0101,Japan
bSanten Pharmaceutical Company Ltd.,40-20,Ofukacho,Kita-ku,Osaka 530-8552,Japan
A R T I C L E I N F O
Article history:
Available online 25 November 2015
Nanocrystal
Wet beads milling
Poorly water soluble compounds
Nanosizing by wet beads milling is one of the methods to improve the solubility of poorly water soluble compounds[1,2]. Hydrophilicpolymers,suchasHydroxypropylcellulose-SSL(HPC), Polyvinylalcohol(PVA),and Polyvinylpyrrolidone-K30(PVP)etc. are used as dispersion stabilizer to prevent aggregation of nanoparticles.However,therearemanycompoundsthatcannot be dispersed by ordinary dispersion stabilizers.In this study, we used Povacoat®as a dispersion stabilizer for several poorly water soluble compounds milled to nanoparticles.The infuences of aggregation of the nanoparticles on the dissolution behaviorandtheoralabsorptionofthecompoundswerestudied using formulations with high and low dispersion characters. In this study,we used various compounds with poorly water solubility such as Griseofulvin(GF),Tolbutamide(TOL),and Hydrochlorothiazide(HYD)etc.The suspension of compound was prepared by milling compound with zirconia beads in polymer aqueoussolutionbyrotation/revolutionpulverizer.Thenpowder formulation of milled compound was prepared by freezedrying the suspension.We compared the effect of Povacoat on dispersion stability of milled compounds with those of PVA, HPC,and PVP as polymers generally used.The dispersion stabilityandcrystallinityofthemilledGFwereevaluatedbyparticles size analyzer,scanning electron microscope(SEM),and powder X-ray diffraction(PXRD).We also evaluated the dissolution behavior and the oral absorption of the milled GF with each polymer.We frst used GF and compared the dispersion stability of the milled GF with Povacoat to those with the other polymers.GF suspension with Povacoat had higher dispersion stability than the suspensions with the other polymers(Fig.1). The re-dispersion stability of the GF powder formulation with Povacoat was higher than those with the other polymer.The aggregation of GF nanoparticles was observed by SEM when PVA,HPC,and PVP were used as dispersion stabilizer.The crystallinityofmilledGFwasfoundtobemaintainedbyPXRD.Milled GF with Povacoat showed higher dissolution behavior and bioavailability compared with the other polymers.The aggregationofnanoparticleshadsignifcantimpactonthedissolution behavior and bioavailability of GF(Fig.2).Povacoat was appliedto TOL and HYD,and the other compounds from the result of GF.In case of both compounds,the formulation with Povacoat had higher dispersion stability than the formulations with the other polymers.It was found that Povacoat could be widely applicable for nanocrystal formulation of poorly water soluble compounds.
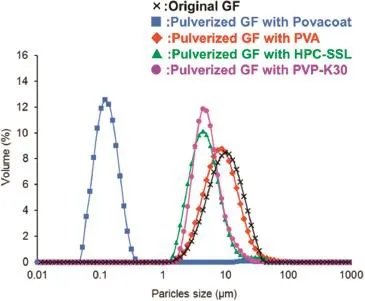
Fig.1–Particle size distribution of the Griseofulvin(GF) suspension after re-dispersing the pulverized GF powder with each polymer in water.
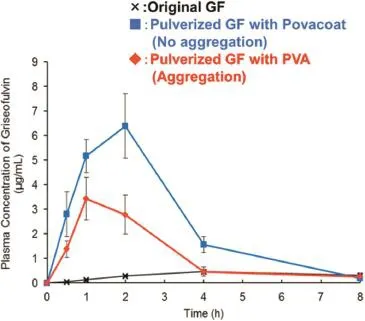
Fig.2–Plasma concentrations of Griseofulvin(GF)after oral administration[3].
Acknowledgements
The authors are grateful to Daido Chemical Corporation(Osaka, Japan)for kindly providing Povacoat.
R E F E R E N C E S
[1]Filippos K,Santipharp P,Yunhui W.Nanosizing–oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev 2007;59:631–644.
[2]Kipp JE.The role of solid nanoparticles technology in the parenteral delivery of poorly water soluble drugs.Int J Pharm 2004;284:109–122.
[3]Yuminoki K,Seko F,Horii S,et al.Preparation and evaluation of high dispersion stable nanocrystal formulation of poorly water-soluble compounds by using povacoat.J Pharm Sci 2014;103(11):3772–3781.
*Corresponding author.Setsunan University,45-1,Nagaotoge-cho,Hirakata,Osaka 573-0101,Japan.
E-mail address:yuminoki@pharm.setsunan.ac.jp(K.Yuminoki).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.10.037
1818-0876/©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
