Hot melt extrusion:An application for enhancing drug solubility
Duangratana Shuwisitkul
Srinakharinwirot University,Nakhon Nayok 26120,Thailand
Conference Abstract
Hot melt extrusion:An application for enhancing drug solubility
Duangratana Shuwisitkul*
Srinakharinwirot University,Nakhon Nayok 26120,Thailand
A R T I C L E I N F O
Article history:
Available online 24 November 2015
Hot-melt extrusion
Advantages
Improvement of drug solubility
Low aqueous solubility of API is a problem of drug product development.There are several methods to enhance drug solubility.Although drugs can increase solubility using many chemical and physical modifcations,the few methods are able to enhance drug solubility for industrial scale[1].Hot-melt extrusion is one reliable process for enhancing drug solubility in a large scale production.It has been recognized as a one step process with several advantages.It can not only increase solubility of drug,but can also be used as a process to prepare controlled release dosage forms.Using a biodegradable polymer with hot-melt extrusion,the drug release of up to 1–6 months can be achieved[2].
Hot-melt extrusion can enhance drug solubility by stabilizing drugs in amorphous form,deaggregating drug particles in a carrier and improving wettability of drugs.A poorly water-soluble drug and a hydrophilic polymer,such as polyvinyl pyrrolidone,are selected and prepared to a solid dispersion using hot-melt extruder.The mixture is extruded to a drug product.An increase in the drug solubility is obtained[3].
One disadvantage of preparing solid dispersion by hot melt extrusion is drug recrystallization.Drugs in amorphous form and dissolved drugs can recrystallize upon storage.Some polymers had the infuence on drug recrystallization.Higher molecular weight of the polymer showed the better inhibition of drug recrystallization.Moreover,the addition of some additives and drug–polymer interaction could extend the drug recrystallization.The inhibition of conversion from amorphous to crystalline drug was observed,thus more stable drug in delivery systems[4].
Acknowledgments
The author acknowledges with thanks the support received from Professor Dr.Isik Özgüney,Professor Dr.Roland Bodmeier and German Academic Exchange Service(DAAD)for their support and encouragement in carrying out this research and work.
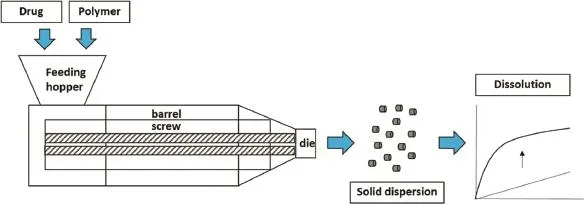
Fig.1–Schematic diagram of hot-melt extrusion process.
R E F E R E N C E S
[1]Kawabata Y,Wada K,Nakatani M,et al.Formulation design for poorly water-soluble drugs based on biopharmaceutics classifcation system:basic approaches and practical applications.Int J Pharm 2011;420:1–10.
[2]Miller DA,McConville JT,Yang W,et al.Hot-melt extrusion for enhanced delivery of drug particles.J Pharm Sci 2007;96:361–376.
[3]Fousteris E,Tarantili PA,Karavas E,et al.Poly(vinyl pyrrolidone)-poloxamer-188 solid dispersions prepared by hot melt extrusion.J Therm Anal Calorim 2013;113:1037–1047.
[4]Fule R,Amin P.Development and evaluation of lafutidine solid dispersion via hot melt extrusion:investigating drugpolymer miscibility with advanced characterization.Asian J Pharm Sci 2014;9:92–106.
*E-mail address:duangrats@g.swu.ac.th.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.10.032
1818-0876/©2016 The Author.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
