Comparison of solid dispersion and nanosuspension for improvement of drug absorption
Qiang Fu,Mengran Guo,Zhonggui He
School of Pharmacy,Shenyang Pharmaceutical University,Shenyang 110016,China
Comparison of solid dispersion and nanosuspension for improvement of drug absorption
Qiang Fu,Mengran Guo,Zhonggui He*
School of Pharmacy,Shenyang Pharmaceutical University,Shenyang 110016,China
A R T I C L E I N F O
Article history:
Available online 23 November 2015
Nanosuspension
Solid dispersion
In vitro
In vivo
Currently,drug discovery technology is heavily dependent on combinatorial chemistry and high throughput screening,resulting in more and more poorly water-soluble drugs,either class II(low solubility and high permeability)or IV(low solubility and low permeability)in the standard biopharmaceutical classifcation system.Owing to the importance of solubility/ dissolution for oral absorption,formulation scientists face a great challenge.Thus,strategies have been proposed to improve the solubility and dissolution rate.Among these approaches, solid dispersion[1]and nanosuspension[2]are the most usually used.However,which one is better for improving the in vitro dissolution and in vivo bioavailability?Some experts have already asked this question and made some efforts to answer it.However,the research carried out to date has involved a limited number of model compounds[3].
The aim of this research is to give a comparison,in terms of the in vitro dissolution and in vivo pharmacokinetics,of solid dispersion and nanocrystals,with different substances (lacidipine,itraconazole,nimodipine,ziprasidone,and etc.)as model compounds.The data are mainly summarized from previous research in our group.The solid dispersions were prepared by the melting method or the solvent method,while the nanocrystals were obtained by media milling or high pressure homogenization.Then,they were characterized by PSD, TEM,DSC,XRD,FTIR,and SEM analysis.Finally,the pharmacokinetic study was carried out in beagle dogs.We found,in most cases,that solid dispersion exhibited higher dissolution profle,while nanocrystals produced the greater increase in oral bioavailability.More importantly,the in vivo pharmacokinetic results did not agree with the in vitro dissolutionprofles.In addition,data in the literatures were also reviewed to support our viewpoint.The results here demonstrate that the use of in vitro dissolution tests for the prediction of in vivo behavior is of limited value for poorly water-soluble drugs when giving comparisons of different formulation strategies.The results obtained in this work will provide guidance for the research and development of poorly water-soluble drugs.
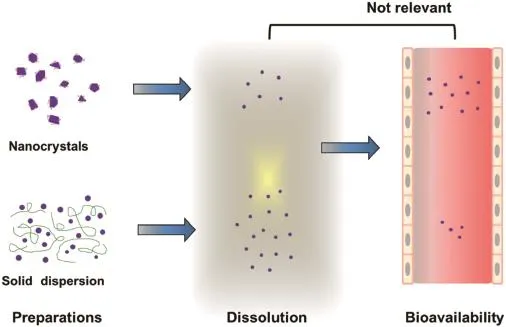
Fig.1–Illustration for the in vitro dissolution and in vivo pharmacokinetic profles for different strategies(solid dispersion, nanocrystals and micronization).Solid dispersion exhibited higher dissolution profle,while nanocrystals produced the greater increase in oral bioavailability.
Acknowledgement
This work was fnancially supported by the National Natural Science Foundation of China(No.81173008).
R E F E R E N C E S
[1]Prudic A,Ji Y,Sadowski G.Thermodynamic phase behavior of API/polymer solid dispersions.Mol Pharm 2014;11:2294–2304.
[2]Rabinow BE.Nanosuspensions in drug delivery.Nat Rev Drug Discov 2004;3:785–796.
[3]Zhang HK,Luo YQ,Yang S,et al.Increased dissolution and oral absorption of itraconazole/Soluplusextrudate compared with itraconazole nanosuspension.Eur J Pharm Biopharm 2013;85:1285–1292.
*E-mail address:hezhonggui@vip.163.com.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.10.009
1818-0876/©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
