Improving Dose Accuracy in Cancer Radiation Therapy Using Deformable Image Registration
Amy Liu, Yadin David, Fred Hosea, Richard Wu
1.Department of Radiation Physics, University of Texas MD Anderson Cancer Center, Houston, Texas, USA; 2.Biomedical Engineering Consultants, LLC, Houston, Texas, USA; 3.Convivia, Oakland, California, USA
Improving Dose Accuracy in Cancer Radiation Therapy Using Deformable Image Registration
Amy Liu1, Yadin David2, Fred Hosea3, Richard Wu1
1.Department of Radiation Physics, University of Texas MD Anderson Cancer Center, Houston, Texas, USA; 2.Biomedical Engineering Consultants, LLC, Houston, Texas, USA; 3.Convivia, Oakland, California, USA
编者按:近年医疗器械产品市场上,得益于计算机技术的普遍应用以及医学影像技术的飞速发展,肿瘤放射治疗、微创手术机器人以及分子影像学等前沿科技均步入了迅猛发展的时代,成为未来最具有发展潜力的几大医学科学前沿领域。前沿技术是高技术领域中具有前瞻性、先导性和探索性的重大技术,是未来高技术更新换代和新兴产业发展的重要基础,是国家高技术创新能力的综合体现。我们邀请到安德森癌症中心的Amy Liu研究员、天津大学的王树新教授以及北京大学人民医院的霍天龙主任就其目前的研究课题分别对精确放疗,基于ARM微控制器的医疗手术机器人以及医学MR分子探针等前沿技术组织一期专栏,向读者全面介绍精准医疗背后的人工智能技术。
ObjectiveTo explore the differences in volume and doses to clinical target volumes (CTVs) and organs at risk (OARs) with and without adaptive treatment plans by using deformable image registration technology.MethodsTen patients with head and neck cancer were selected for this retrospective study. Each patient's original treatment plan was generated using the Eclipse treatment planning system (Varian, Inc.). Verif cation CT scans were performed during the third week of treatment. The verif cation CT images were registered with the original CT images using the Eclipse rigid registration tool simulating daily patient treatment alignment. Then, deformable image registrations (Velocity, Inc.) were performed between the two CT image sets, and the CTVs and major OARs were transferred from the original CT images to the verif cation CT images. The original treatment plan was then copied into the verif cation CT image set to calculate the radiation dose ref ecting the most recent anatomic changes. Verif cation plan doses were evaluated by a radiation oncologist, who determined whether an adaptive treatment plan was required. We compared the accumulated doses to CTVs and OARs between the original and adaptive plans, as well as between the adaptive and verif cation plans, to simulate the doses that would have been delivered if the adaptive plans were not used. All dosimetric data were extracted using the Eclipse Application Programming Interface tool, which was developed in house to access the Eclipse database. Results Body contours were different after 3 weeks of treatment. Mean volumes of all CTVs were reduced (P≤0.04), and the volumes of left and right parotid glands decreased (P≤0.004). There were no significant differences in the volumes of brainstem and oral cavity (P≥0.14) between the original and verif cation CT scans. The spinal cord had a mean 8.7% decrease in volume (P=0.04). Mean doses of CTVs were all decreased (P≤0.04), whereas the mean doses of the right parotid and oral cavity were increased (P=0.03).ConclusionVerif cation CT scans and adaptive planning are required during the course of proton therapy for patients with head and neck cancer to identify anatomic and dosimetric changes and to ensure adequate doses to target volumes and safe doses to normal tissues. Our results indicate that deformable image registration can serve as an essential tool for current proton treatment.
deformable image registration; proton IMPT for head and neck cancer; adaptive plan; dose uncertainty
There has been substantial growth in the use of proton therapy in the treatment of cancer in the past decade[1]. Owing to the sharp distal falloff of proton beams within tissue, this technologically advanced therapy has substantial advantages over conventional photon therapy, reducing unnecessaryradiation doses to organs at risk (OARs) and healthy tissue. Numerous reports have documented the theoretical advantages of proton therapy over photon therapy for head and neck malignancies[2-3], and clinical results achieved with proton beams have been impressive[4]. The fundamental tenet of radiotherapy is the delivery of a high radiation dose to the tumor while limiting the dose to the surrounding normal tissues[5]. However, because changes in patient anatomy (such as weight loss) occur during treatment (usually by 5~7 weeks) and because protons have a def ned range to target, the planned radiation dose to clinical target volumes (CTVs) and dose to OARs may be changed signif cantly.
Deformable (non-rigid) imaging registration (DIR) has gained popularity in recent years, becoming an essential tool in both adaptive radiation therapy and image-guided radiation therapy to account for tissue changes during the course of treatment[6]. Using deformable image registration, we can evaluate the dose precisely throughout the entire treatment and, if needed, generate an adaptive treatment plan to correct dose def ciencies and ensure that OAR doses are appropriate. Numerous studies have been performed using deformable dose accumulation on prostate and head and neck cancer treatments with photon IMRT modality[7-8]and for adaptive therapy[6,9]. However, there have been no studies demonstrated the degree of dose variations for patients treated with IMPT modality. Because of the sharp falloff in proton distal range, small changes in anatomy can cause dosimetric changes in target and OARs. Wang et al[10]found head and neck cancer patients could have anatomical structure changes during the course of radiotherapy owing to the shrinkage of the tumor or lymph nodes or to body weight loss. It was also found that gross tumor volumes (GTVs) can be reduced by as much as 70%[11]. Therefore, it is important during the proton treatment to evaluate the dosimetric effect.
In this retrospective study, we evaluated the contribution of repeat CT verif cation scans and adaptive IMPT re-planning in assessing anatomic volumetric and dosimetric changes during the course of proton treatment.
1 MATERIALS AND METHODS
1.1 Patient selection, planning methods, and treatment delivery
This study included 10 patients with head and neck squamous cell carcinoma. These patients had been treated with a definitive chemo-radiation protocol using IMPT to the GTV with a dose of 70 Gy (relative biological equivalence, REB) in 2 Gy per fraction. The clinical target volumes (CTV1, CTV2 and CTV3) represented tissues considered to be at risk of microscopic disease but not gross disease, including lymph node regions. The average volumes were 176, 204, and 241 cm3for CTV1, CTV2, and CTV3, respectively. CT scans and volumes were obtained in a GE CT simulator and transferred electronically to the treatment planning system (TPS) at the proton therapy center of MD Anderson Cancer Center. Each patient's original treatment plan was generated using the Eclipse treatment planning system (Varian, Inc.). Verification CT scans were performed during the third week of treatment. The verification CT images were registered with the original planning CT images using the Eclipse rigid registration tool simulating daily patient treatment alignment. All plans were calculated using the TPS for delivery of proton therapy with discrete spot beam scanning[12]. The proton therapy center uses a synchrotron and the Hitachi Probeat proton beam therapy system (Hitachi, Ltd., Tokyo, Japan). A three-field beam arrangement was used, with right anterior oblique (RAO), left anterior oblique (LAO), and posterior to anterior (PA) direction beams. The f elds were all non-coplanar, as shown in Figure 1.

Figure 1 Verif cation CT scan. a. Shows an overview of the noncoplanar field directions; b. Illustrates skin location changes (2~5 mm) noted after 3 weeks of proton therapy treatment in a representative patient with head and neck cancer.
The maximum energy of the proton beams per field varied between 102 and 203 MeV, depending on the case and the incident beam angle. An energy absorber (6.7 cm water equivalent thickness) was mounted on the treatment snout, to engage the system to treat targets in the shallow area of the head and neck.
Deformable image registrations were performed betweenthe two CT image sets using commercial deformable registration software (Velocity, Inc.). The accuracy of the deformable image registration algorithms was previously evaluated by Kirby et al[13]. The treatment targets had three CTVs (CTV1, CTV2, and CTV3) corresponding to intended cobalt equivalent dose levels of 70, 63, and 57 Gy, respectively. Major OARs for the study were the parotids, oral cavity, brainstem, and spinal cord. Both CTVs and OARs were deformed and transferred from the original CT images to the verification CT image data set[14]. All deformed contours were reviewed by a radiation oncologist before generating the adaptive plan. The original treatment plan was then copied into the verif cation CT image set to calculate the radiation dose ref ecting the most recent anatomic changes. The verification plan was evaluated and compared with the original approved plan on all dosimetric matrixes by a radiation oncologist, who determined whether an adaptive treatment plan was required. We compared the accumulated dose to CTVs and OARs between the original plan and the adaptive plan, as well as between the adaptive plan and verif cation plan, to simulate the doses that would have been delivered if the adaptive plans were not used. Among this selected group of patients, we used the last nine fractions of thirty three fractions total for adaptive plan.
1.3 Volume and dose comparisons
All CTVs and OAR volumes were compared between the original and verification CT scans with paired-sample analysis. Again, the OARs analyzed were the spinal cord, brainstem, parotid glands, and oral cavity. For each plan, dose volume histograms (DVHs) were calculated for CTVs and OARs. First, the original plan (based on the original CT) was compared with the adaptive plan (based on the verification CT) with the renormalized 33-fraction treatment dose to ensure that the plans were consistent in their quality. Then, comparisons were made among verif cation and adaptive plans. This comparison accounted for the last nine fractions delivered by the adaptive plan. The selected nine fractions for the adaptive plan were based on the weekly CT verification image and plan review results.
All dosimetric data were extracted using the Eclipse Application Programming Interface (API) tool, which was developed in house at M.D. Anderson to access the Eclipse database. (The API tool facilitates efficient and accurate extraction of dosimetric data from the Eclipse treatment planning database, compared with manual data collection using the tools within the Eclipse system.)
Wilcoxon matched-pairs nonparametric tests were used to evaluate the effect of adaptive plan vs. verif cation on volume and plan dosimetric changes. A probability value of ≤0.05 was considered signif cant. All statistics were calculated using R.
2 RESULTS
2.1 Volume comparisons
As illustrated in Figure 1 (which shows a representative image from one patient), body contours were changed after 3 weeks of treatment. Also, the CTV and OAR locations inside of patients had some degree of change. As a result, the dose distribution changed. Figure 2 illustrates the CTV dose coverage changes between the original and verif cation CT scans. Table 1 compares the volumes of CTVs and OARs for the original vs. verification CT scans. Between the original and verification CT scans, the mean volumes of all CTVs were reduced (P≤0.04), and the volumes of left and right parotid glands decreased (P≤0.004). There were no signif cant differences in the volumes of the brainstem or oral cavity (P≥0.14) between the original and verif cation CT scans. The spinal cord had a mean 8.7% decrease in volume (P=0.04). Our f ndings were consistent with those of previous studies that found that CTV and parotid volumes shrink after radiation treatment (in this case, after 3 weeks). Volumes of the other OARs examined (brainstem, spinal cord, and oral cavity) remained relatively consistent.

Figure 2 Example of the base of tongue of a patient with carcinoma. a. From the original plan; b. From a verification plan. The figure shows the dose distribution changes due to slight changes in the tongue location.

Table 1 Volume comparisons
2.2 Dosimetric comparisons
There were no signif cant dosimetric differences betweenthe original plan and adaptive plan (Figure 3), which could be attributed to the re-planning process with the same planning goal setting that should achieve adaptive plan dose constraint as close as possible using verif cation CT.
1.利润追求。组建联合体的根本驱动在于外部交易内部化、生产经营规模化和标准化,提高经济效益,产生合作剩余,成员共同协商收益分配,使各方能够获取比单独经营时更多的利润。农村产业融合强调的是不同产业相互渗透、交叉、重组后,经营主体由竞争关系转变为合作关系,实现范围经济,使各主体获得比独立经营某一产业时更多的利润。二者都是借由市场主体联结,降低交易费用,只是前者侧重规模经济性,后者更凸显范围经济性。

Figure 3 Dose volume histograms (DVHs). a. For clinical target volume (CTV) comparison and are derived from original plan (square lines) and adaptive plan (triangles) in the head and neck case; b. For CTVs and organs at risk (OARs) derived from the verification plan (square lines) and adaptive plan (triangle lines). There was no significant difference for CTV coverage between the original plan and the adaptive plan. There was a significant difference between the verification plan and adaptive plan.
Figure 3 shows a selected patient DVH for CTVs and OARs derived from the adaptive plan (triangles) and verif cation plan (squares). On verif cation plan DVH, 95% CTV1 volume was covered by the prescription dose (70 Gy); however, the dose homogeneity and conformity[15]degraded. The decreases in dose homogeneity and conformity were more signif cant for CTV2 and CTV3. There were increased doses in the right parotid and oral cavity. Values for V26(the percentage volume that received more than 26 Gy) for the right parotid were 33% and 54%, respectively. For the oral cavity, V30values were 6% and 10%, respectively.
However, there were significant dosimetric differences
between the adaptive plan and verification plan. As shown in Table 2, on verif cation, the mean doses (D99and D95) of CTV1, CTV2, and CTV3 were all decreased (P≤0.04), whereas the mean doses (Dmean) of the right parotid and oral cavity were increased (P=0.03). Our study also indicated that there was no signif cant mean dose (Dmean) increase to the left parotid (P=0.4) and mean maximum dose (Dmax) increases to brainstem (P=0.28) and spinal cord (P=0.12).
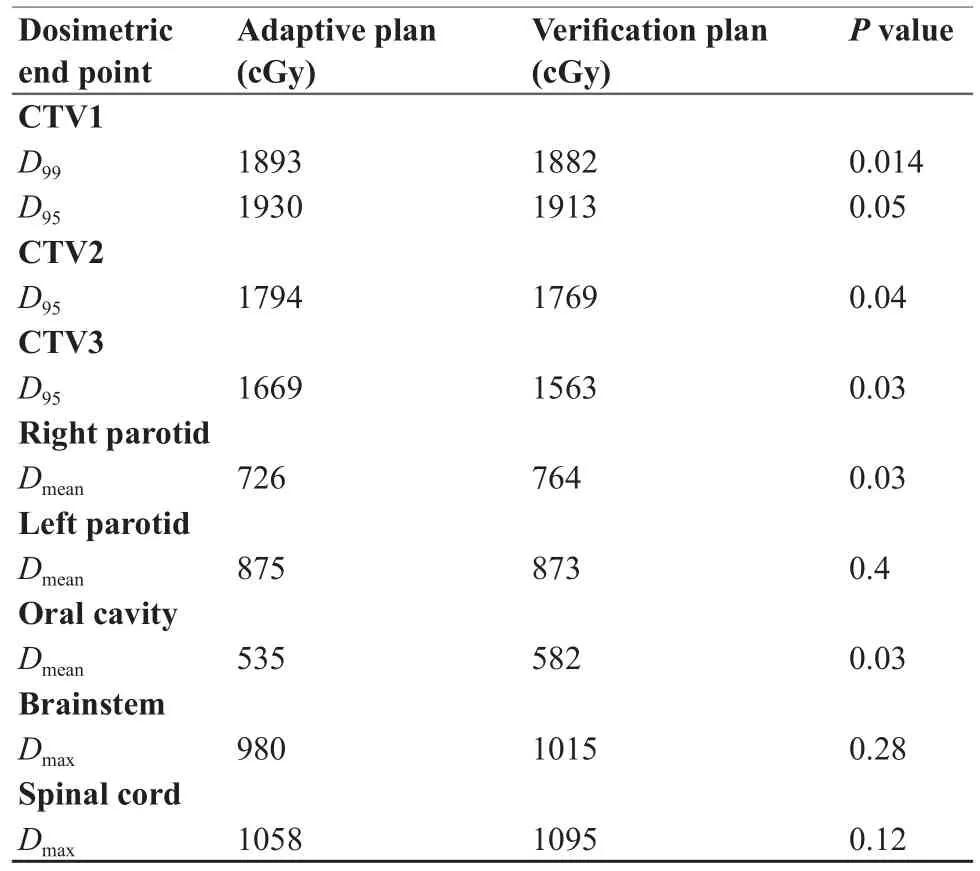
Table 2 Dosimetric comparison of adaptive plan with verif cation plan
3 DISCUSSION
This retrospective study demonstrated the importance of performing verification CT imaging and the need for adaptive planning during the course of IMPT for patients with head and neck cancer. The dosimetry outcomes reported in this study are consistent with those of other investigations[16]without use of DIR. Deformable image registration technology has played an important role in generating adaptive treatment planning. It can reduce the workload of physicians in re-contouring all structures in the new CT image data set, which is time consuming but a very important step to ensure the accuracy of the adaptive treatment plan designs. In this study, the spinal cord deformation volume should not change theoretically between the two CT data sets, but results of the statistical analysis suggest that it had more changes than expected (P=0.04). This could be related to two reasons. First, the deformed spinal cord contours on the verification CT scans were not reviewed as rigorously as were CTVs and parotids. Second, the spinal cord (a soft tissue structure) is inside the C spine vertebra (a hard bony structure). There may be deformation inaccuracy by the Velocity DIR algorithm that may focus more on high-density areas. On the other hand, there were no signif cantvolume changes for the brainstem or oral cavity. The low-level dose to these two structures may also have contributed to these results. Many commercial and in-house DIR systems have been developed in recent years. These systems use a variety of approaches, and the accuracy of contour deformation needs to be further evaluated and used with precaution[13]. As a result, the new contours review or moderation process on the new CT data set cannot be completely eliminated and replaced with DIR.
The results here of parotid dose changes are consistent with the finding from Hansen et al[16]. The right parotid mean dose was increased on verification compared with the adaptive plan (P=0.03), whereas the left parotid dose had insignif cant changes (P=0.4). Among the ten patients, four patients had GTV on the right side and six on the left. Further studies are needed to evaluate the geometric relationship of deformed CTVs and parotids.
Future investigation can also be extended to a large number of patients being treated that have used DIR to obtain accumulated doses from multiple plans to correlate treatment outcomes. Future studies should also investigate change in CTVs and different degrees of shrinkage in OARs (especially parotids) between photon and proton modalities that can be uniquely due to proton variable RBE to a variety of different types of tissue in which DIR may have increased uncertainty to perform contour deformation.
There are increased workloads and costs for physicians, physicists, and dosimetrists to perform reimaging and re-planning. The Eclipse API may automate this process in a seamless fashion and thus reduce the burden on all involved personnel.
4 CONCLUSION
Verif cation CT imaging and adaptive planning are required during the course of IMPT for patients with head and neck cancer to identify anatomic and dosimetric changes and to ensure adequate doses to target volumes and safe doses to normal tissues. Our results indicate that deformable image registration can serve as an essential tool for current IMPT regimens.
5 ACKNOWLEDGMENT
We thank Michael Worley and the Department of Scientif c Publications at MD Anderson Cancer Center for editorial assistance.
[1] Smith AR.Proton therapy[J].Phys Med Biol,2006,51(13): R491-504.
[2] Lomax AJ,Goitein M,Adams J.Intensity modulation in radiotherapy:photons versus protons in the paranasal sinus[J]. Radiother Oncol,2003,66(1):11-18.
[3] Steneker M,Lomax A,Schneider U.Intensity modulated photon and proton therapy for the treatment of head and neck tumors[J]. Radiother Oncol,2006,80(2):263-267.
[4] Frank SJ,Cox JD,Gillin M,et al.Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice[J].Int J Radiat Oncol Biol Phys,2014,89(4):846-853.
[5] Njeh CF,Dong L,Orton CG.Point/Counterpoint.IGRT has limited clinical value due to lack of accurate tumor delineation[J]. Med Phys,2013,40(4):040601.
[6] Lawson JD,Schreibmann E,Jani AB,et al.Quantitative evaluation of a cone-beam computed tomography-planning computed tomography deformable image registration method for adaptive radiation therapy[J].J Appl Clin Med Phys,2007,8(4):2432. [7] Cui Y,Piper JW,Harrison AS,et al.Deformable Dose Accumulation with Image Guided Radiotherapy for Final Dose Evaluation in Pelvic Cases[J].J Nucl Med Radiat Ther,2012,(Suppl.3):1.
[8] Veiga C,McClelland J,Moinuddin S,et al.Toward adaptive radiotherapy for head and neck patients: Feasibility study on using CT-to-CBCT deformable registration for“dose of the day”calculations[J].Med Phys,2014,41(3):031703.
[9] Chitapanarux I,Chomprasert K,Nobnaop W,et al.A dosimetric comparison of two-phase adaptive intensity-modulated radiotherapy for locally advanced nasopharyngeal cancer[J].J Radiat Res,2015,56(3):529-538.
[10] Wang W,Yang H,Hu W,et al.Clinical study of the necessity of replanning before the 25thfraction during the course of intensitymodulated radiotherapy for patients with nasopharyngeal carcinoma[J].Int J Radiat Oncol Biol Phys,2010,77(2):617-621.
[11] Barker JL Jr,Garden AS,Ang KK,et al.Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system[J].Int J Radiat Oncol Biol Phys, 2004,59(4):960-970.
[12] Gillin MT,Sahoo N,Bues M,et al.Commissioning of the discrete spot scanning proton beam delivery system at the University of Texas M.D.Anderson Cancer Center,Proton Therapy Center, Houston[J].Med Phys,2010,37(1):154-163.
[13] Kirby N,Chuang C,Ueda U,et al.The need for applicationbased adaptation of deformable image registration[J].Med Phys,2013,40(1):011702.
[14] Schreibmann E,Pantalone P,Waller A,et al.A measure to evaluate deformable registration fields in clinical settings[J].J Appl Clin Med Phys,2012,13(5):3829.
[15] Iqbal K,Isa M,Buzdar SA,et al.Treatment planning evaluation of sliding window and multiple static segments techniquein intensity modulated radiotherapy[J].Rep Pract Oncol Radiother,2012,18(2):101-106.
[16] Hansen EK,Bucci MK,Quivey JM,et al.Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer[J]. Int J Radiat Oncol Biol Phys,2006,64(2):355-362.
R197.39 [Document code] A
10.3969/j.issn.1674-1633.2016.11.002 [Article ID] 1674-1633(2016)11-0009-05
Received: 2015-09-09
Amy Liu, Computational Scientist, Department of Radiation Physics, Pickens Academic Tower, 1400 Pressler St., Unit 1420, Houston, Texas 77030-3722, USA. E-mail: AYLiu@mdanderson.org

