香豆素改性透明质酸颗粒乳化剂的制备及应用
诸 超, 朱 叶, 魏 玮, 顾 瑶, 刘晓亚
(江南大学化学与材料工程学院, 食品胶体与生物技术教育部重点实验室,江苏 无锡 214122)
香豆素改性透明质酸颗粒乳化剂的制备及应用
诸 超, 朱 叶, 魏 玮, 顾 瑶, 刘晓亚
(江南大学化学与材料工程学院, 食品胶体与生物技术教育部重点实验室,江苏 无锡 214122)
利用7-(2-羟基乙氧基)-4-甲基香豆素(HEMC)疏水改性透明质酸(HA)制得光敏双亲大分子(HA-HEMC),并通过自组装形成了HA-HEMC胶体粒子。通过核磁共振氢谱和紫外分光光度计确定了HA-HEMC的结构及取代度;采用动态激光光散射、纳米粒度仪和透射电镜等手段对胶体粒子的性质及形貌进行了表征。进一步以HA-HEMC胶体粒子作为颗粒乳化剂稳定油水界面,研究了胶体粒子质量浓度、油相类型、水相pH和盐浓度对胶体粒子乳化性能的影响。结果表明:HA-HEMC可以在选择性溶剂中自组装形成粒径约为236 nm的球形胶体粒子;该胶体粒子能够在较宽的pH范围内(3~11)稳定水包油型乳液且所得乳液有良好的耐盐性;此外,HA-HEMC胶体粒子还能稳定多种油-水体系。
透明质酸疏水改性; 光敏双亲大分子; 自组装胶体粒子; 颗粒乳化剂
由固体颗粒代替传统小分子表面活性剂稳定油-水界面所制备的乳液被称为Pickering乳液,此类具有表面活性的固体颗粒被称为颗粒乳化剂[1]。相较于传统的小分子表面活性剂,颗粒乳化剂具有乳化效率高、稳定性好、毒性低以及无分子迁移等优点[2],在食品[3-4]、化妆品、生物医用、石油开采以及新材料制备[5-6]等领域具有一定的应用前景。目前被广泛报道的颗粒乳化剂主要包括无机纳米颗粒[7]、表面改性或有机-无机杂化纳米粒子[8]、聚合物乳胶粒子(Latex)[9]、聚合物微凝胶[10]以及大分子自组装胶体粒子[11]等。其中,大分子自组装胶体粒子因其良好的分子可设计性和结构可控性成为了最有前景的颗粒乳化剂之一。
2005年,Fujii等[12]首次报道了由三嵌段聚合物聚环氧乙烷-b-聚甲基丙烯酸单甘油酯-b-聚二甲胺乙基甲基丙烯酸酯(PEO-b-PGMA-b-PDEA)自组装形成的壳交联胶体粒子为颗粒乳化剂稳定十一醇-水体系,并通过pH调控实现了乳液的循环乳化-破乳-再乳化。本课题组前期也在以双亲性无规聚合物自组装胶体粒子为颗粒乳化剂稳定Pickering乳液方面做了大量的研究[13-16]。然而,目前所报道的大分子自组装胶体粒子颗粒乳化剂大多基于合成聚合物,在一定程度上限制了其在生物医药、食品、化妆品等领域的应用。因此,发展由环境友好且安全无毒性的天然材料所制备的颗粒乳化剂体系具有十分重要的意义。最近,基于天然多糖(如壳聚糖、纤维素以及淀粉等)的颗粒乳化剂成为了人们关注的热点。Liu等[17]利用碱性条件下的壳聚糖为颗粒乳化剂稳定O/W型乳液; Rojas等[18]利用N-异丙基丙烯酰胺改性的纤维素为温敏性颗粒乳化剂稳定O/W型乳液;Tan等[19]利用乙酸酐和邻苯二甲酸酐疏水改性的淀粉为颗粒乳化剂稳定甘油三酯-水乳液。
透明质酸(HA)是一种具有良好生物相容性的直链线型阴离子酸性黏多糖,由2个双糖单位D-葡萄糖醛酸及N-乙酰葡糖胺为重复单元组成,广泛分布于软结缔组织细胞的外基质中[20-21]。HA具有良好的水溶性和卓越的保湿性能,因而常用于高级化妆品[22]与保健品中,且HA可以进行多种化学修饰从而使其功能化,是极具潜力的生物医药材料[23-25],受到广大科研工作者关注。目前,国内外对于HA的研究大多集中于组织工程与药物负载领域,基于HA的颗粒乳化剂的制备及其稳定油水界面的研究尚鲜有报道。本文选择含有光敏基团的7-(2-羟基乙氧基)-4-甲基香豆素(HEMC)作为疏水单体对HA进行疏水改性,制得双亲性天然大分子HA-HEMC;并在选择性溶剂中诱导疏水改性的HA自组装形成胶体粒子,并以此胶体粒子作为颗粒乳化剂稳定油水界面,研究了胶体粒子质量浓度、油相类型、水相pH以及盐浓度对胶体粒子乳化性能的影响。本文一方面拓展了大分子自组装颗粒乳化剂的种类,为制备低生物毒性的Pickering乳液提供了全新的思路;另一方面胶体粒子中的疏水单元HEMC中含有光敏单元香豆素,其在赋予HA疏水性的同时提供了一定的紫外吸收性,为Pickering乳液的功能化和在化妆品领域的实际应用开辟了新的研究思路。
1 实验部分
1.1 试剂
透明质酸钠(HA-Na):化妆品级,黏均分子量为1.1×104,镇江东元生物科技有限公司;IR-120阳离子交换树脂(H型):百灵威试剂公司;四丁基氢氧化铵(TBA-OH):w=25%,上海麦恪林生化科技有限公司;N,N-二环己基碳酰亚胺(DCC):化学纯,国药集团化学试剂有限公司;4-二甲氨基吡啶(DMAP,纯度为99%)、2-溴乙醇(纯度为96%):阿拉丁试剂有限公司;7-羟基-4-甲基香豆素(AMC):纯度为97%,阿法埃莎试剂有限公司;二甲亚砜(DMSO)、丙酮、乙醇、乙醚、无水碳酸钾、无水硫酸镁:分析纯,国药集团化学试剂有限公司;白油(26#):化妆品级,上海文华化工颜料有限公司。
1.2 HA-HEMC的合成
将5.0 g(22.71 mmol)AMC、5.0 g(40.01 mmol)2-溴乙醇、3.0 g碳酸钾加入到含50 mL乙醇的三口圆底烧瓶中,在85 ℃下回流24 h。反应结束后,用水溶解碳酸钾,溶液用乙醚萃取2次。在有机层加入适量无水硫酸镁干燥,抽滤除去硫酸镁。通过旋蒸除去溶剂,真空干燥即可得到HEMC[26]。
称取0.5 g(1.08 mmol)HA与四丁基氢氧化铵的复配物(HA-TBA)溶于10 mL无水DMSO中,待完全溶解后加入0.66 g(3.19 mmol)DCC和0.26 g(2.16 mmol)DMAP,在60 ℃下搅拌2 h以活化羧基;随后称取1.07 g(4.86 mmol)HEMC加入到上述反应液中,在60 ℃下反应48 h。反应结束后,抽滤除去不溶物,滤液用丙酮进行沉淀、离心,此过程重复2次。离心得到的固体用10~15 mL DMSO溶解,转移至透析袋,用氯化钠溶液透析(截留分子量为3 500)1 d,再用去离子水透析以除去未反应的单体和杂质。冷冻干燥即可得到HA-HEMC,其合成路线如图1所示。
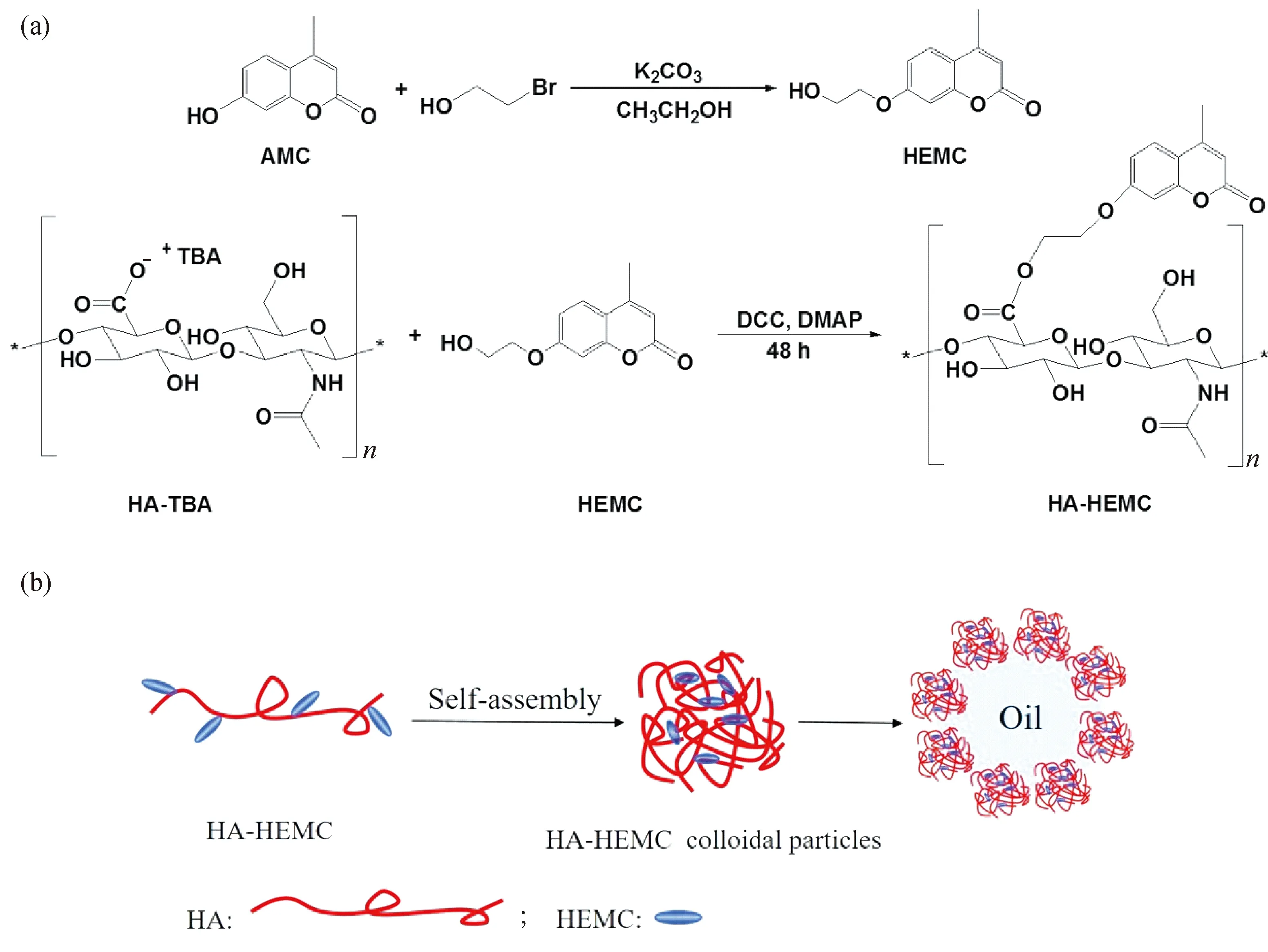
图1 HA-HEMC的合成(a)以及HA-HEMC胶体粒子的制备和胶体粒子在油水界面的行为示意图(b)Fig.1 Schematic illustration of synthesis of HA-HEMC(a) and the perparation and the oil-in-water interfacial behavior of HA-HEMC colloidal particles(b)
1.3 HA-HEMC胶体粒子的制备及其在油水界面的行为
将HA-HEMC 溶于无水DMSO得到质量浓度为10 mg/mL的溶液,以15 μL/min的速率将超纯水滴加到1 mL的HA-HEMC溶液中,利用紫外分光光度计追踪HA-HEMC混合溶液在621 nm处吸光度随水含量增加的变化,当达到临界水含量时(即吸光度发生突跃时的水含量),制得HA-HEMC胶体粒子。
以HA-HEMC胶体粒子分散液作为水相,取3 mL胶体粒子分散液与油相等体积混合,室温下使用XHF-D高速分散器以20 500 r/min的转速均质2 min,得到目标乳液,HA-HEMC胶体粒子稳定油水界面的示意图如图1所示。
1.4 表征
采用德国Bruker公司AVANCE Ⅲ型核磁共振仪对HA、HEMC与HA-HEMC样品的结构进行表征,其中HEMC与HA-HEMC以氘代DMSO为溶剂,HA以D2O为溶剂;用北京普析通用公司TU-1901 型紫外分光光度计测试HA-HEMC的紫外吸收谱图,观察HA-HEMC的紫外吸收区域;采用德国ALV公司ALV/DLS/SLS-5022F动态激光光散射(DLS)测定胶体粒子的流体动力学半径(Rh)及其分布(f(Rh));用美国布鲁克海文仪器公司Zeta PALS 型纳米粒度仪和 Zeta 电位仪测定胶体粒子的粒径分布以及Zeta电位;用日本JEOL电子株式会社JEOL JEM-2100 型透射电镜(TEM)观察胶体粒子的形貌;乳液放置24 h后,采用日本尼康株式会社Nikon P500型数码相机拍摄乳液的表观照片;用中国Motic公司DM-BA 450型光学显微镜观察乳液类型及乳液滴大小。
2 结果与讨论
2.1 HEMC的结构表征
图2为HEMC的核磁共振氢谱图。1H-NMR:2.10(s,3H,—CH3),3.75(t,2H,—CH2OR),4.10

图2 HEMC的核磁共振氢谱图Fig.2 1H-NMR spectra of HEMC
(t,2H,—CH2OAr),6.15(s,H,香豆素3位上—CH—),6.7~7.0(m,2H,香豆素5、6位上—CH—CH—),7.6(s,H,香豆素8位上—CH—),HEMC的核磁氢谱与文献[26]报道一致,证明成功制备了HEMC。
2.2 HA-HEMC的结构表征
HA和HA-HEMC的1H-NMR谱图如图3所示,与未改性的HA相比,HA-HEMC的1H-NMR谱图在化学位移7~8 处出现了香豆素苯环的质子峰,在化学位移4.1~5.2 处出现了香豆素侧链亚甲基的质子峰,证明已成功制备HA-HEMC。利用HA-HEMC上苯环的质子峰面积与HA中糖苷键上甲基的质子峰面积的积分比计算得到HA-HEMC的取代度为22%。
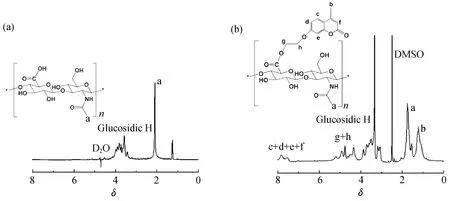
图3 HA(a)和HA-HEMC(b)的核磁共振氢谱图Fig.3 1H-NMR spectra of HA(a) and HA-HEMC(b)

图4 HA、HEMC和HA-HEMC的紫外谱图Fig.4 UV spectra of HA,HEMC and HA-HEMC
图4为HA、HEMC以及HA-HEMC的紫外吸收谱图。由于HEMC含有苯环以及不饱和双键,在323 nm处有较强的紫外吸收峰[27],HA-HEMC在320 nm处也出现紫外吸收峰,而HA在此区间无紫外吸收峰,进一步证明了HA的成功改性,且改性后的HA具有紫外吸收特性。
2.3 HA-HEMC胶体粒子的表征
HA-HEMC溶液的吸光度随选择性溶剂DMSO-H2O中水的体积分数(φ(H2O))增加的变化,如图5(a)所示。当φ(H2O)达到29.1%时,HA-HEMC通过疏水作用自组装形成胶体粒子。通过DLS和TEM对胶体粒子的尺寸和形貌进行表征,结果如图5(b)所示。HA-HEMC胶体粒子呈单峰分散状态,其平均粒径约为236 nm。TEM显示胶体粒子呈球形,粒径为220~250 nm,与DLS显示的粒径相符。
由于HA-HEMC含有羧基基团,其胶体粒子对pH应具有响应性。图6(a)是胶体粒子在不同pH下粒径的变化,可以观察到,胶体粒子的Rh随着pH的增加而呈现逐渐增大的趋势。这主要是因为,随pH的增加,羧基的去质子化程度不断增强,使HA-HEMC链段的荷电量不断增加,增强了链段之间的静电排斥力,从而使胶束结构变得越来越松散,导致Rh增大[28],从而也证实了上述羧基去质子化使得胶体粒子荷电量增加的结论。
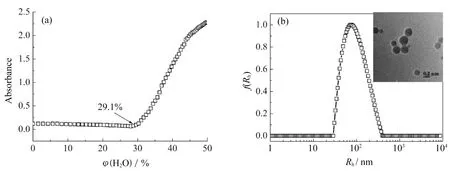
图5 HA-HEMC溶液的吸光度随φ(H2O)的变化(a);HA-HEMC胶体粒子的流体力学半径及其分布(b)
盐对HA-HEMC胶体粒子性质的影响如图6(b)所示。HA-HEMC胶体粒子表面带有负电荷的羧酸根,当c(NaCl)=0.15 mol/L时,胶体粒子的Zeta电位值增加,胶体粒子的Rh减小,这是由于盐的加入产生静电屏蔽作用导致胶体粒子的荷电量降低,亲水链段之间静电斥力下降,使分子链更易卷曲得到Rh较小的胶体粒子[29]。当c(NaCl)>0.15 mol/L时,随着盐浓度的增加,胶体粒子的Zeta电位值逐渐趋于零,胶体粒子之间的静电斥力较小使其更易聚集,Rh逐渐增加。

图6 HA-HEMC胶体粒子的粒径和Zeta电位随pH(a)和盐浓度(b)的变化Fig.6 Particle size and Zeta potential of HA-HEMC colloidal particles with different pH value(a) and salt concentration(b)
利用紫外分光光度计追踪不同UV(365 nm)光照时间下胶体粒子的紫外吸收,从而验证HA-HEMC的光敏性。由图7(a)和7(b)可见,随着UV光照时间的增加,胶体粒子在325 nm左右的紫外吸收强度逐渐减弱,这是由于香豆素基团发生了光二聚反应。在最初的5 min光照时间下,光二聚现象最明显,当时间到达115 min时,由于胶体粒子核内的香豆素基团基本完成光二聚(HA-HEMC的光二聚示意图如图7(c)所示),进一步的UV光照对其紫外吸收基本无影响[30]。
2.4 HA-HEMC胶体粒子的乳化性能研究
不同质量浓度的HA-HEMC胶体粒子分散液与白油等体积混合、均质后的照片如图8所示。当ρ(HA-HEMC)<0.2 mg/mL时,无法形成稳定的乳液;当ρ(HA-HEMC)≥0.2 mg/mL时,可以获得稳定的水包油(O/W)型乳液;且随着ρ(HA-HEMC)的增加,其乳液层高度逐渐增加,乳液滴的粒径逐渐减小。这是由于当ρ(HA-HEMC)过低时,吸附于乳液滴表面的胶体粒子数量过少,无法在油水界面形成致密的粒子膜,导致乳液滴的聚并或直接破乳;当ρ(HA-HEMC)增加,胶体粒子在油水界面可形成较致密的粒子膜,有效地阻碍了乳液滴的聚并,有利于得到乳液滴粒径较小的乳液[29]。
6种不同极性的油相与HA-HEMC胶体粒子分散液等体积混合、均质后的照片如图9所示。随着油相极性的增加,胶体粒子的乳化能力变差。这是由于随着油相极性的增加,胶体粒子在油水界面的表面张力减小,胶体粒子从油水界面脱吸所需的能量下降,不利于形成稳定的乳液[31]。当以1-辛醇作为油相时,由于其黏度较大,减缓了乳液滴聚并的速率,使其形成稳定性较差的乳液,大约放置7 d后破乳。

ρ(HA-HEMC)/(mg·mL-1):a-0.1;b-0.2;c-0.3;d-0.5;e-1.0;pH=5.22图8 HA-HEMC胶体粒子稳定白油的表观照片(A)和显微镜照片(B)Fig.8 Appearance of emulsions(A) and optical microscope images(B) of the emulsion droplets stabilized by HA-HEMC aqueous solution with different mass concentrations
以HA-HEMC胶体粒子为对象,水相的pH对胶体粒子乳化性能的影响如图10所示。HA-HEMC胶体粒子在较宽的pH范围内均可稳定白油形成O/W型乳液;且随着pH的增加,乳液滴的粒径逐渐减小。这一方面是由于羧基的逐渐电离,使得乳液滴之间的静电斥力逐渐增加,有效地阻碍了乳液滴的聚并;另一方面是胶体粒子粒径逐渐增加,溶胀的胶体粒子在均质过程中易发生变形,使其在油水界面具有较大的界面面积,有利于胶体粒子稳定油水界面[32]。
利用不同盐浓度的HA-HEMC胶体粒子分散液为水相,探讨了水相的盐浓度对胶体粒子乳化性能的影响,结果如图11所示。当c(NaCl)=0.15 mol/L时,由于胶体粒子的粒径较小,有利于获得乳液滴粒径较小、分布均一的乳液;随着盐浓度的增加,胶体粒子表面的荷电量降低,乳液滴之间的静电斥力较小,使得乳液滴易聚并,故而使乳液滴粒径增加;但是当c(NaCl)=0.6 mol/L时,其乳液滴粒径变小,这是由于此时胶体粒子大量聚集,胶体粒子分散液呈弱絮凝状态[33],有利于胶体粒子稳定油水界面。

ρ(HA-HEMC)=0.5 mg/mL;pH=5.22;Oil types:a-Cyclohexane,b-Liquid paraffin,c-Toluene,d-Dichloromethane,e-Ethyl acetate,f-1-Octanol图9 HA-HEMC胶体粒子稳定不同极性油相的表观照片(A)和乳液滴的显微镜图片(B)Fig.9 Appearance of emulsions(A) and optical microscope images(B) of the emulsion droplets stabilized by HA-HEMC aqueous solution with different oil types
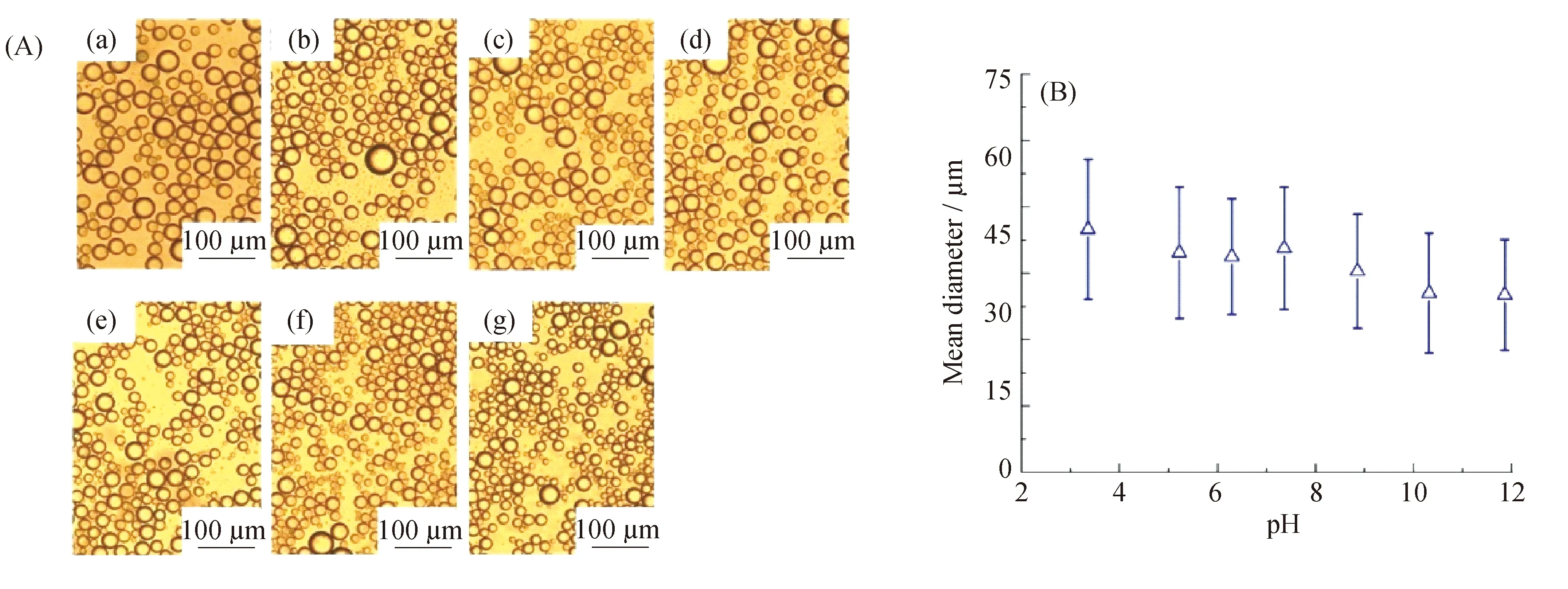
ρ(HA-HEMC)=0.5 mg/mL;pH:a-3.60,b-5.59,c-7.22,d-9.35,e-11.26,f-10.32,g-11.86图10 不同pH条件下,HA-HEMC胶体粒子稳定白油的显微镜照片(A)以及乳液滴的平均粒径(B)Fig.10 Optical microscope images(A) and the mean diameter(B) of emulsion droplets stabilized by HA-HEMC aqueous solution with different pH values

ρ(HA-HEMC)=0.5 mg/mL;pH=5.22;c(NaCl)/(mol·L-1):a-0,b-0.15,c-0.3,d-0.45,e-0.6图11 不同盐浓度下,HA-HEMC胶体粒子稳定白油的显微镜照片(A)以及乳液滴的平均粒径(B)Fig.11 Optical microscope images(A) and the mean diameter(B) of emulsion droplets stabilized by HA-HEMC aqueous solution with different concentrations of NaCl
3 结 论
光敏双亲大分子HA-HEMC可通过疏水作用自组装形成球形胶体粒子。该胶体粒子作为颗粒乳化剂可制备O/W型的Pickering乳液,与以前研究的天然大分子颗粒乳化剂对比,所需颗粒乳化剂浓度较小,具有较大的应用优势。该胶体粒子在较宽的pH范围内(3~11)可稳定白油-水体系,且所得乳液具有良好的耐盐性及长期稳定性;此外,该胶体粒子具有良好的普适性,可长效稳定多种油-水体系。
[1] PICKERING S U.Cxcvi.—Emulsions[J].Journal of the Chemical Society,Transacyions,1907,91:2001-2021.
[2] MORSEA J,DUPIN D,THOMPSON K L,etal.Novel Pickering emulsifiers based on pH-responsive poly(tert-butylaminoethyl methacrylate) latexes[J].Langmuir,2012,28(32):11733-11744.
[3] YAN Mao,LIU Fu,TANG Chuanhe.Properties and microstructure of transglutaminase-set soy protein-stabilized emulsion gels[J].Food Research International,2013,52(1):409-418.
[4] LIU Fu,TANG Chuanhe.Phytosterol colloidal particles as Pickering stabilizers for emulsions[J].Journal of Agricultural and Food Chemistry,2014,62(22):5133-5141.
[5] SHAHIDAN N N,LIU Ruixue,THAIBOONROD S,etal.Hollow colloidosomes prepared using accelerated solvent evaporation[J].Langmuir,2013,29(45):13676-13685.
[6] OH B H L,BISMARCK A,PARK M B C.High internal phase emulsion templating with self-emulsifying and thermoresponsive chitosan-graft-PNIPAM-graft-oligoproline[J].Biomacromolecules,2014,15(5):1777-1787.
[7] MURAKAMI R,MORIYAMA H,NOGUCHI T,etal.Effects of the density difference between water and oil on stabilization of powdered oil-in-water emulsions[J].Langmuir,2014,30(2):496-500.
[8] ZHANG Qing,BAI Ruixue,GUO Ting,etal.Switchable Pickering emulsions stabilized by awakened TiO2nanoparticle emulsifiers using UV/dark actuation[J].ACS Applied Materials and Interfaces,2015,7(33):18240-18246.
[9] MA Chunfeng,BI Xiaobo,NGAI To,etal.Polyurethane-based nanoparticles as stabilizers for oil-in-water or water-in-oil Pickering emulsions[J].Journal of Materials Chemistry A,2013,1:5353-5360.
[10] MORSE A J,ARMES S P,THOMPSON K L,etal.Novel Pickering emulsifiers based on pH-responsive poly(2-(diethylamino) ethyl methacrylate) latexes[J].Langmuir,2013,29(18):11733-11744.
[11] SUN Jianhua,YI Chenglin,WEI Wei,etal.Nanohybrids from direct chemical self-assembly of poly(styrene-alt-maleic anhydride) as pH-responsive particulate emulsifiers[J].Langmuir,2014,30(49):1-28.
[12] FUJII Syuji,CAI Yuanli,WEAVER J V M,etal.Syntheses of shell cross-linked micelles using acidic ABC triblock copolymers and their application as pH-responsive particulate emulsifiers[J].Journal of the American Chemical Society,2005,127(20):7304-7305.
[13] YI Chenglin,LIU Na,ZHENG Junchao,etal.Dual-responsive poly(styrene-alt-maleic acid)-graft-poly(N-isopropyl acrylamide) micelles as switchable emulsifiers[J].Journal of Colloid and Interface Science,2012,380:90-98.
[14] LIU Xiaoya,YI Chenglin,ZHU Ye,etal.Pickering emulsions stabilized by self-assembled colloidal particles of copolymers of P(St-alt-MAn)-co-P(VM-alt-MAn)[J].Journal of Colloid and Interface Science,2010,351(2):315-322.
[15] YI Chenglin,YANG Yiqun,ZHU Ye,etal.Self-assembly and emulsification of poly {[styrene-alt-maleic acid]-co-[styrene-alt-(N-3,4-dihydroxyphenylethyl-maleamic acid)]}[J].Langmuir,2012,28(25):9211-9222.
[16] WEI Wei,WANG Ting,YI Chenglin,etal.Self-assembled micelles based on branched poly(styrene-alt-maleic anhydride) as particulate emulsifiers[J].RSC Advances,2015,5(2):1564-1570.
[17] LIU Hao,WANG Chaoyang,ZOU Shengwen,etal.Simple,reversible emulsion system switched by pH on the basis of chitosan without any hydrophobic modification[J].Langmuir,2012,28(30):11017-11024.
[18] ZOPPEJ O,VENDITTI R A,ROJAS O J.Pickering emulsions stabilized by cellulose nanocrystals grafted with thermo-responsive polymer brushes[J].Journal of Colloid and Interface Science,2012,369(1):202-209.
[19] TAN Ying,XU Kun,NIU Cheng,etal.Triglyceride-water emulsions stabilised by starch-based nanoparticles[J].Food Hydrocolloids,2014,36:70-75.
[20] MARTINS Madalena,AZOIA N G,SHIMANOVICH U,etal.Design of novel BSA/hyaluronic acid nanodispersions for transdermal pharma purposes[J].Molecular Pharmaceutics,2014,11(5):1479-1488.
[21] UNALG C,CUTTICA D,ANNABI N,etal.Synthesis and characterization of hybrid hyaluronic acid-gelatin hydrogels[J].Biomacromolecules,2013,14(4):1085-1092.
[22] 董鹏程,董少雄,陈少婷,等.透明质酸(HA)在化妆品方面的作用功效以及应用[J].科技资讯,2013(30):75-76.
[23] RODELL C B,WADE R J,PURCELL B P,etal.Selective proteolytic degradation of guest-host assembled,injectable hyaluronic acid hydrogels[J].ACS Biomaterials Science and Engineering,2015,1(4):277-286.
[24] LEE H,LEE K,PARK T G.Hyaluronic acid-paclitaxel conjugate micelles:Synthesis,characterization,and antitumor activity[J].Bioconjugate Chemistry,2008,19(6):1319-1325.
[25] YU Meihua,JAMBHRUNKAR S,THORN P,etal.Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells[J].Nanoscale,2013,5(1):178-183.
[26] JIANG Jinqiang,QI B,LEPAGE M,etal.Polymer Micelles stabilization on demand through reversible photo-cross-linking[J].Macromolecules,2007,40(4):790-792.
[27] MADDIPATLA M V S N,WEHRUNG D,TANG Chuan,etal.Photoresponsive coumarin polyesters that exhibit cross-linking and chain scission properties[J].Macromolecules,2013,46(13):5133-5140.
[28] BINKS B P,MURAKAMI R,ARMES S P,etal.Effects of pH and salt concentration on oil-in-water emulsions stabilized solely by nanocomposite microgel particles[J].Langmuir,2006,22(5):2050-2057.
[29] BINKSB P.Particles as surfactants—similarities and differences[J].Current Opinion in Colloid and Interface Science,2002,7(1-2):21-41.
[30] 林海芳,易成林,徐晶,等.壳聚糖的光敏改性及其胶束化行为[J].日用化学工业,2012,42(3):167-191.
[31] GILLES S,FREDERIQUE H P,GILLES P.Dispersibility and emulsion-stabilizing effect of cellulose nanowhiskers esterified by vinyl acetate and vinyl cinnamate[J].Biomacromolecule,2013,14(8):2937-2944.
[32] ZHANG Cuige,ZHU Ye,ZHANG Rongli,etal.Pickering emulsions stabilized by composite nanoparticles prepared from lysozyme and dopamine modified poly(γ-glutamic acid):Effects of pH value on the stability of the emulsion and the activity of lysozyme[J].RSC Advances,2015,5:90651-90658.
[33] AVEYARD R,BINKS B P,CLINT J H.Emulsions stabilised solely by colloidal particles[J].Advances in Colloid and Interface Science,2003,100-102:503-546.
Preparation and Application of Particulate Emulsifier of Coumarin Modified Hyaluronic Acid
ZHU Chao, ZHU Ye, WEI Wei, GU Yao, LIU Xiao-ya
(Key Laboratory of Food Colloids and Biotechnology of Ministry of Education, School of Chemical andMaterial Engineering,Jiangnan University, Wuxi 214122, Jiangsu, China)
By using 7-(2-hydroxyethoxy)-4-methylcoumarin(HEMC) hydrophobic modified hyaluronic acid (HA), photosensitive amphiphilic macromolecules(HA-HEMC) were prepared and HA-HEMC colloidal particles were obtained by self assembly.The structure and substitution degree of HA-HEMC were characterized by hydrogen nuclear magnetic spectra and UV-Vis spectrophotometer.The properties and morphologies of HA-HEMC colloidal particles were characterized by dynamic light scattering,nanoparticle size analyzer and transmission electron microscopy.Additionally,HA-HEMC colloidal particles were applied as the particulate emulsifier to stabilize oil-water interface and the emulsification performance of HA-HEMC colloidal particles in different conditions,such as mass concentration of colloidal particles,oil type,pH value and salt concentration in aqueous phase were investigated.Results showed that HA-HEMC were able to form spherical colloidal particles with the average diameter of 263 nm in selective solvent and HA-HEMC colloidal particles could stable oil-in-water Pickering emulsions in the broader range of pH(3~11) and various concentrations of NaCl.In addition,the colloidal particles were able to stabilize variety of oil-water system.
hydrophobic modified hyaluronic acid; photosensitive amphiphilic macromolecule; self-assembled colloidal particle; particulate emulsifier
1008-9357(2016)04-0388-009
10.14133/j.cnki.1008-9357.2016.04.003
2016-08-23
国家自然科学基金 (20974041,21174056) ;江南大学食品胶体与生物技术教育部重点实验室开发基金 (JDSJ2014-07)
诸 超(1992-),女,江苏苏州人,硕士生,研究方向为功能高分子自组装和胶体界面化学。E-mail:zhuchaojn@163.com
刘晓亚,E-mail:lxy@jiangnan.edu.cn
O636.9

