Gum arabic improves semen quality and oxidative stress capacity in alloxan induced diabetes rats
Jaafar S Fedail,Abdelkareem A Ahmed, Hassan H Musa, Elsadik Ismail, Amal Z Sifaldin, Taha H Musa
1Department of Biology, Faculty of Education, University of Nyala, Sudan
2Key Laboratory of Animal Reproduction, College of Animal Science and Technology, Nanjing Agricultural University, Nanjing, China
3Department of Physiology and Biochemistry, Faculty of Veterinary Science, University of Nyala,Sudan
4Department of microbiology, Faculty of Medical Laboratory Sciences, University of Khartoum, Sudan
5Department of Anatomy, Faculty of Veterinary Science, University of Nyala, Nyala, Sudan
6Department of Molecular Genetics, Institute of Molecular Biology, University of Nyala,Sudan
7Department of Epidemiology, School of Public Health, Southeast University, Nanjing, 210095, China
Gum arabic improves semen quality and oxidative stress capacity in alloxan induced diabetes rats
Jaafar S Fedail1,2*,Abdelkareem A Ahmed3*, Hassan H Musa4, Elsadik Ismail5, Amal Z Sifaldin6, Taha H Musa7
1Department of Biology, Faculty of Education, University of Nyala, Sudan
2Key Laboratory of Animal Reproduction, College of Animal Science and Technology, Nanjing Agricultural University, Nanjing, China
3Department of Physiology and Biochemistry, Faculty of Veterinary Science, University of Nyala,Sudan
4Department of microbiology, Faculty of Medical Laboratory Sciences, University of Khartoum, Sudan
5Department of Anatomy, Faculty of Veterinary Science, University of Nyala, Nyala, Sudan
6Department of Molecular Genetics, Institute of Molecular Biology, University of Nyala,Sudan
7Department of Epidemiology, School of Public Health, Southeast University, Nanjing, 210095, China
ARTICLE INFO
Article history:
Received 2016
Received in revised form 2016
Accepted 2016
Available online 2016
Gum arabic
Oxidative stress
Rat
Testis
Type I diabetes
Objective:To explore effect of gum arabic (GA) on semen quality and oxidative stress capacity of alloxan induced diabetes rats.Methods:In this study, male Sprague-Dawley rats were divided into 3 groups (n=20 of each): control group, diabetic group which were injected with allaoxan, and diabetic group which was given 10% GA in the form of drinking water for 10 weeks. The effect of GA on testicular oxidative stress and sperm quality were investigated. Testicular antioxidant was detected by the measurement of antioxidant enzymes, malondialdehyde in testis tissue. Moreover, plasma lipids, testis histopathological changes and oxidative stress related genes mRNA were evaluated.Results:The treatment of GA significantly (P<0.05) increased semen quality compared the diabetic and control groups. Similarly, the treatment of GA significantly (P<0.05) increased the activities of catalase, superoxide dismutase and glutathione peroxidase compared to diabetic and control groups. The treatment of GA significantly (P<0.05) decreased testis malondialdehyde, plasma total cholesterol, low-density lipoprotein cholesterol and triglyceride concentrations, whereas increased high-density lipoproteincholesterol concentrations compared to the diabetic groups. Glutathione peroxidase and superoxide dismutase mRNA expression were significantly (P<0.05) increased in GA treated group compared to diabetic and control groups. All testes of diabetic rats displayed obvious degeneration; whereas slight degeneration was seen in GA treated rats when compared to diabetic control group.Conclusions:Our findings imply that GA may protect testis via enhancement of antioxidant capacity, it may be useful to meliorate the diabetic fertility complications.
1. Introduction
Natural substances have been used as a source of medicinal treatments for several decades [1,2], and plants-based products play a critical role in the treatment of diabetes mellitus (DM) globally [3]. In underdeveloped and developing countries worlds, herbal medicine is considered as a traditional medicine for treatment of diabetes [4]. The worldwide increased infertility or sterility rates have been a hotly debated problem [5], mainly on the comparative contributions of obesity and metabolic disorder factors [6,7]. Infertility is an important clinical problem, affecting people psychosocially [8] and medically [9]. In recent years, oxidative stress has been implicated in the progression of male infertility [10]. The experimental evidence has been implicated that these damages are caused by free radicals[11]. The deleterious effects of oxidative stress results from either an increased production of react reactive oxygen species [12] or a decreased natural cell antioxidant capacity of an organism [13]. However, the utilization of foods rich in antioxidant phytochemicals may decrease the harmful effects caused by oxidative damage in several tissues including liver, intestine and kidney[14].
DM is a metabolic disorder characterized by high blood glucose levels due to the defects in the secretion of insulin and its action or both [15]. The chronic hyperglycemia is linked with protracted dysfunction, damage, and collapse of functioning of a variety of organs, including kidneys[16], nerves, heart[17], and blood vessels[18] and testis[19,20]. Hyperglycaemia generates reactive oxygen species[21], which sequentially cause cell damage via different pathways [22-24]. The damage of the cells ultimately results in the secondary complications of DM[25]. Numerous studies from the diabetic patients and experimental animals confirmed that sustained hyperglycemia resulted in the reduction of reproductive performance[26-28]. Since high blood glucose probably lead to the oxidative stress and cellular apoptosis[29,30], which in turn lead to the structural and functional impairments[27] and finally contribute to infertility[31,32]. Recent studies has broken the age factor in DM as it diagnosed both in younger and overage persons[33]. Therefore, diabetes-induced reproductive dysfunction is emerging as a new and urgent challenge [27]. The molecular mechanism through which diabetes induces male infertility is not fully understood.
Many experimental and clinical reports have been conducted on the molecular mechanisms responsible for the changes induced by DM in reproductive system of male but much remains to be clarified [34]. Some studies implicated that the diabetes induced male infertility through histological damage of the epididymis[28], decreased sperm motility[35], semen volume[36], sperm counts, motility and morphology [37] and disruption of seminiferous tubular morphology[38]. Moreover, DM induced male infertility via decreasing serum levels of luteinizing hormone, follicular stimulating [39] and testosterone[40].
Gum arabic (GA) is an edible, dried sticky exudate from Acacia seyal and Acacia senegal, which is rich in soluble dietary fiber. It is universally used in food manufacturing and pharmaceutical preparations as preservative and emulsifier[41]. In the Middle East and North Africa, it has been given orally as traditional medicine by different communities for centuries[42]. GA has been used to decrease both frequency and need of hemodialysis in patients who suffer with chronic renal failure[43]. It has powerful antioxidant properties, and used to decrease the experimental nephrotoxicity induced by gentamicin[43], cisplatin[44] and to decrease cardiotoxicity [45]. Moreover, GA is reported to reduce oxidative and inflammation against adenine induced chronic renal failure in rats[46] and improved the kidney functions in diabetic rat[47,48]. Yet, the effects of GA on oxidative stress in testis of type I diabetic rats have not been conducted. Moreover, it is less clear whether GA can alter oxidative related enzymes activity and genes expression in testis of type I a diabetic rat.
Therefore, in the current experiment, we used type I diabetic rat model to examine our assumption that the treatment of GA in the form of drinking water may decrease the oxidative damage in the testis, and the reduction of oxidative stress may associate with alteration of oxidative related genes expression in tests.
2. Materials and methods
2.1. Animals and experimental protocol
Male Sprague-Dawley rats 90 d of age, weighing (200±10) g were purchased from Shanghai Laboratory Animal Center, Chinese Academy of Science and were housed under controlled environment with a 12 h light-dark cycle. The rats were adapted for one week prior to start the study and provided free access to water and standard rat rations throughout the experimental period. The rats were then divided into 3 groups: control group (n=20) provided standard animal pellet and water ad labitum; diabetic group, intraperitoneal alloxan injected (n=20); and diabetic group (n=20) offered 15% GA in drinking water for 8 weeks. The GA was obtained from Sudanese Company for GA (Khartoum, Sudan). The dose of GA and the time duration was chosen based on our previous studies[49]. Type I DM was induced as described by Adeyiet al[50]. Briefly, alloxan monohydrate was purchased from Sigma-Aldrich China (Shanghai, China), and type I DM was induced by single intraperitoneal injection of 150 mg/kg of alloxan monohydrate dissolved in normal saline after an overnight fast. Surviving rats after 3 d that have blood glucose levels more than 200 mg/dL were classified as type I diabetic models rat, were used for further study. All diabetic rats were euthanized after 8 weeks of treatment. The animals were fasted overnight, blood samples were collected prior to euthanasia. Body weights and organ weights were measured; blood and tissue samples were collected and kept at -80 ℃for mRNA expression analysis.
2.2. Assessment of testis oxidative stress
Lipid peroxidation in testis was assessed by measuring the amount of malondialdehyde (MDA) as described by Bloomet al[51] using obtainable commercial MDA kit (Nanjing Jiancheng Bioengineering Company, Nanjing, China). The MDA was measured in a UV spectrophotometry at 532 nm as described in the manufacturer’s instructions. Approximately, 0.5 g of testis tissues were homogenized in 4.5 mL of ice-cold PBS buffer for preparation of testis homogenate, the homogenates were then centrifuged for 10 min at 3 000 r/pm and the supernatantwas kept at -20 ℃ until analyzed. The levels of MDA in the tissue were expressed as nmol/g tissue.
Glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) kits were purchased from a commercial company (Nanjing Jiancheng Bioengineering Company, Nanjing, China). About 1 g of testis tissues were cut into small pieces then homogenized in ice-cold normal saline (0.85%, pH = 7.4) (1:9, wt/ v) with an Ultra-Turrax (T8, IKA-labortechnik Staufen, Germany). Testis homogenates were centrifuged at 1 000gfor 15 min at 4 ℃, and the supernatants were collected. The supernatants were used for the assays of SOD, GPx, CAT and GSH. SOD activity was measured as described by Cohenet al[52]. The specific activity was expressed in terms of units for milligrams of protein. The activities of GPx and CAT were assayed by the methods described by Cohenet al. [53], and Pagliaet al. [54], respectively.
2.3. Serum lipid profile and blood glucose
Serum lipid profile including triglyceride (TG), high-density lipoproteincholesterol (HDL-c), low-density lipoprotein cholesterol (LDL), and blood glucose were measured using an OLYMPUS AU400 chemistry analyzer (Nanjing Military Hospital, Nanjing, China). The levels of hepatic TG were measured in tissue homogenates. TG concentration measured using a tissue TG assay kit.
2.4. Sperm analysis
Testes with epididymis were removed, and the caudal epididymidis were separated from the testis and the semen was collected. Squeezed semen was incubated in buffer containing BSA at 37 ℃ for 30 min. The normal morphology of sperm, motility, sperm count and its viability were measured in groups of experimental rats. We used Makler Chamber and light microscopy (Olympus Co., Tokyo, Japan) for sperm movement analysis. The motility was expressed as the percentages of progressive motility including rapid (Grade a) and slow (Grade b) spermatozoa, non-progressive (Grade c) and immotile (Grade d) spermatozoa. The morphology of the spermatozoa was evaluated using the original dilution for motility, diluted 1:20 with 10% neutral buffered formalin. The sperms were classified according to the presence of one or more abnormal features such as tail defect (colloid, irregular, short, or multiple tails); neck and middle piece (bend middle piece, distended irregular, abnormality thin middle piece); and head defects (small or large size, round head, double or detached head). The data were presented as percentage of morphological normal sperm.
2.5. Histopathology examinations
Testis samples were fixed immediately after animal euthanized in paraformaldehyde solution and embedded in paraffin, sectioned consecutively at 4 µm. The sections then stained with hematoxylin and eosin to examine the morphological changes in diabetic, diabetic rats treated with GA and compare with control. Slides at every time-point were stained with hematoxylin and eosin and observed under a light microscope (Nikon, Tokyo, Japan).
2.6. RNA extraction and real-time PCR
About 150 mg of testis was ground in liquid N2, and a portion of about 50 mg was used for the extraction of total RNA using TRIzol total RNA kit (Invitrogen, Biotechnology Co, Ltd, Carlsbad, CA, USA) according to the manufacturer’s instruction. Two approaches were taken in account to ensure that all the total RNA preparations are free of genomic DNA contamination. Firstly, total RNAs were treated with 10 U DNase I (RNase Free, D2215, Takara, Japan) for 30 min at 37℃, and purified according to the manufacturer’s protocol. Secondly, the primers for the reference gene (β-actin) were designed to span an intron, so any genomic DNA contamination could be reported easily with an extra product in the melting curves for real-time PCR. Realtime PCR was performed in Mx3000P (Stratagene, USA) according to our previous publications [55]. Mock RT and No Template Controls were included to monitor the possible contamination of genomic and environmental DNA at both RT and PCR steps. The pooled sample was made by mixing equal quantity of RT products (cDNA) from all samples, and was used for optimizing the PCR condition and tailoring the standard curves for each target gene. The melting curves were performed to insure a single specific PCR product for each gene. The PCR products were sequenced to validate the identity of the amplicons. Primers specific for SOD, CAT and GSH-Px (Table 1) was synthesized by Geneary (Shanghai, China). Rat β-actin was used as a house keeping gene for normalization purpose. The method of 2-ΔΔCtwas used to analyze the real-time PCR data [56]. The mRNA abundances were presented as the fold change relative to the average level of the control group.
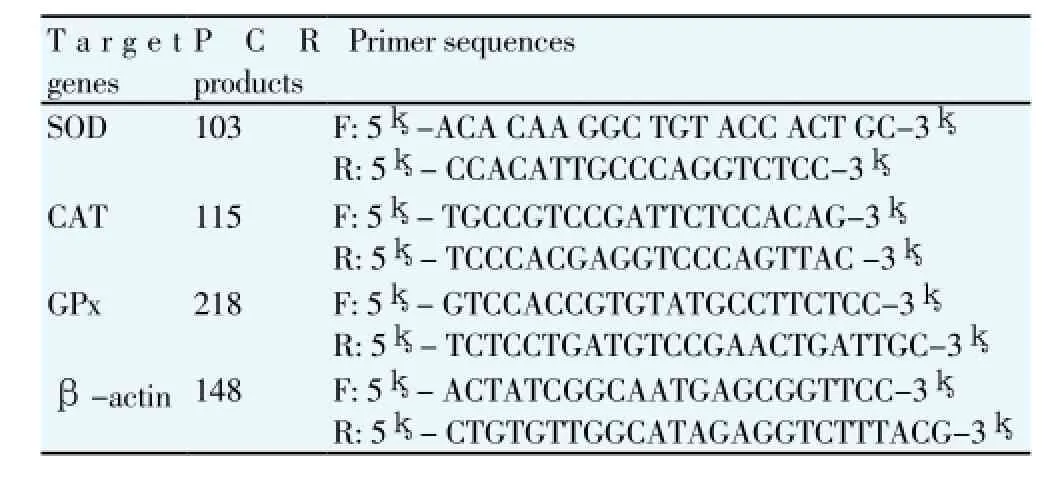
Table 1Real-time PCR primers.
2.7. Statistical analysis
Descriptive statistics was performed to ensure the normality and homogeneity of variances prior to analyses of parameters. Body weight, organs weight, activities of antioxidative enzymes, lipid peroxidation, as well as the relative quantitative data of mRNA expression were analyzed by one-way ANOVA using SPSS 16.0 for Windows, followed by a least-significant difference (LSD) test for individual comparisons. AP-value <0.05 was considered as significant difference.
3. Results
3.1. Effect of GA treatment on body weight and organs weight
In this study, no significant differences were observed in final body weight in diabetic rat or diabetic rat treated with GA groups when compared to the control group. Additionally, no significant differences were found in epididymis weight and testis weight among all groups regarding the treatment of GA (Table 2).
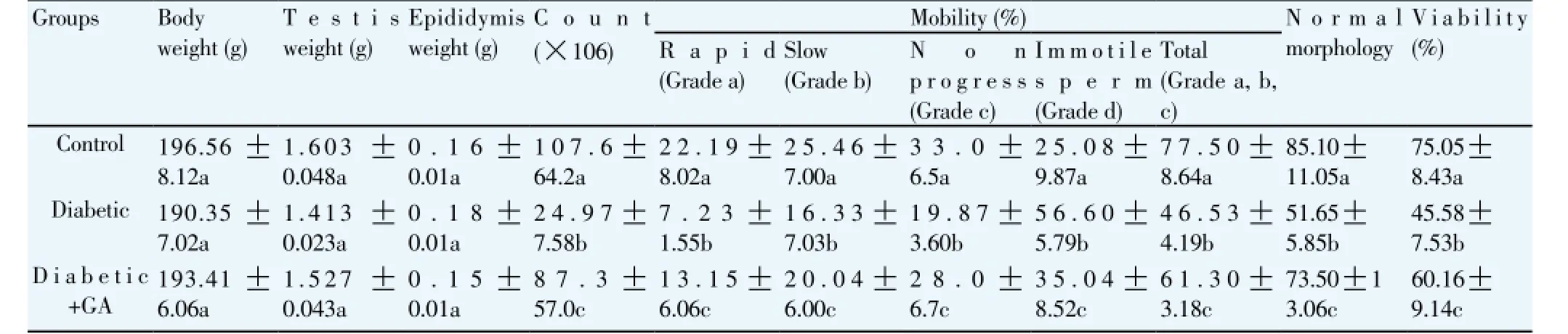
Table 2Effect of GA treatments on body weight, testis weight, epididymis weight and semen quality parameters.
3.2. Plasma lipids and glucose concentrations
Plasma TG and LDL-c concentrations were significantly (P<0.05) higher in diabetic rat group compared to the control group. GA treatment significantly (P<0.05) decreased plasma TG, LDL-c concentrations when compared to the diabetic rat group. In contrast, the treatment of GA significantly increased plasma HDL-c concentration compared to the diabetic rat group. Furthermore, GA treatment significantly (P<0.05) decreased plasma glucose concentrations compared to the diabetic rat group (Table 3).
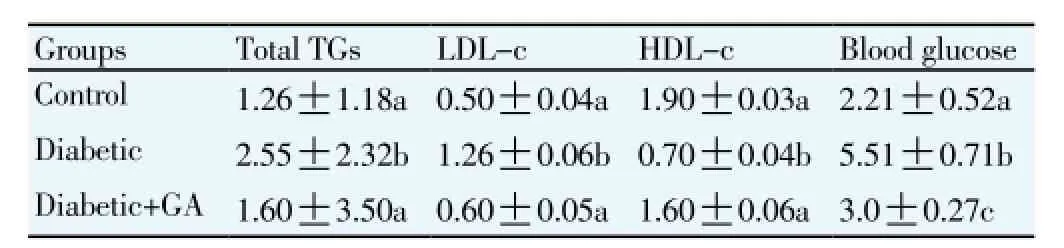
Table 3Effect of GA on plasma lipid profile and blood glucose concentrations of diabetic rat (g/L).
3.3. Effect of GA treatment on semen quality
Sperm count, sperm rapid mobility, slow motility, non progress mobility, total motility and sperm vitality were significantly (P< 0.05) decreased in diabetic rat group compared to the control. The treatment of GA significantly (P<0.05) increased the above sperm parameters. On the other hand, alloxan induced diabetes significantly increased immotile sperm compared to the control group. The treatment of GA significantly (P<0.05) reduced immotile sperm compared to that in diabetic rat (Table 2).
3.4. Effect of GA treatment on testicular oxidative stress
In this study, the MDA levels were significantly (P<0.05) higher in the diabetic rat group compared to the control group. The treatment of GA significant (P<0.05) reduced MDA levels in testis compared to that of diabetic rats (Table 4). In contrast, the diabetic rat group showed the reduced testicular activities of CAT, SOD and GPx. However, the treatment of GA significantly (P<0.05) increased testicular activities of CAT, GPx and SOD compared to the diabetic rat group (Table 4).
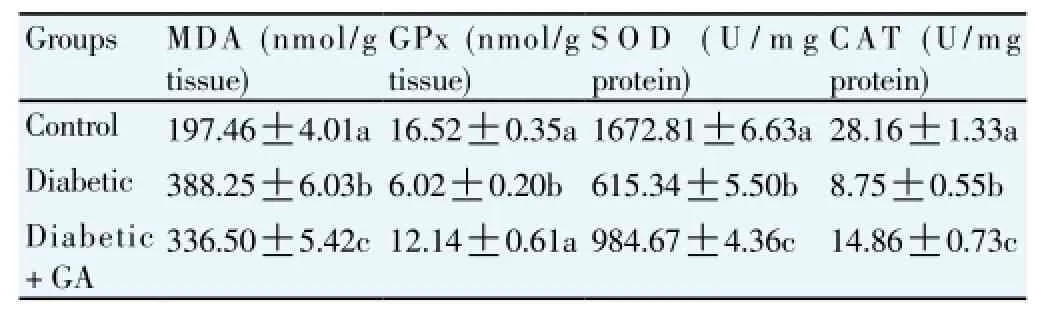
Table 4Effect of GA treatments on antioxidant enzymes activities and lipid peroxidation in the testis.
3.5.Histopathological change
Histological profile in testis of the control group demonstrated normal features of the testis without any visible degenerative changes (Figure 1A). All testes of diabetic rats showed an obvious degeneration with severe vacoulations (Figure 1B). However, the treatment of GA significantly protected the testis of diabetic rats from degeneration and vacoulations compared to control and diabetic rats (Figure 1C).

Figure 1. Effect of GA treatments testis histopathology, control (A), diabetic (B), and diabetic treated with GA (C).
3.6. Antioxidant genes mRNA expression in testis
In the present study, the treatment of alloxan decreased testicular mRNA expression of SOD (Figure 2A) and GPx (Figure 2B) compared to the control group. The treatment of GA significantly (P<0.05) increased testicular mRNA expression of GPx and SOD compared tothe diabetic rats. Unlikely, no significant differences were observed in testicular mRNA expression of CAT in all groups regardless of GA treatment (Figure 2C).

Figure 2.Effect of GA treatments on testis mRNA expression of SOD (A), GPx (B), and CAT (C). Bars with different letters are significantly different atP<0.05.
4. Discussion
GA has been reported to have protective effects in several diseased conditions, including nephrotoxicity[43,57], cardiotoxicity[45], inflammation[46] and DM[48]. In our previous study, we observed that GA protected ovary from oxidative stress damage in mice fed with high fat diet[58]. Here we reported that the treatment of GA increased sperm quality in diabetic rat. Our findings are in line with previous report that ginger (Zingiber officinale) a dietary fibre improved reproductive function by increased testis weights, increased semen quantity and motility in diabetic rat[59].
Dietary fiber consumption has been associated with a decrease in lipids and carbohydrate both in health and diabetes patients [60,61]. The treatment of dietary fibre reduced the levels of serum LDL-c and TGs in diabetic rat[62]. In addition, the dietary fibre was reported to have potential hypoglycaemic effects by decreasing blood glucose in diabetic rabbit[63]. In harmony with these results, we reported that the treatment of GA significantly reduced plasma TG, LDL-c and plasma glucose concentrations in diabetic rat. A variety of mechanisms have been projected to explain the hypocholesterolemic effect of the dietary fibre [64-66]. Some reports have indicated that the viscosity of soluble and fermentable dietary fibers contribute considerably in lipid lowering effects both in humans and animals[65,67]. Although other reports proposed that this property may not associated to plasma lipids[68]. The most mechanism which clearly implicated is that GA enhanced fecal bile acid excretion and neutral sterol excretion or alterations in lipid digestion and absorption[69,70].
Experimental studies reported that the induction of diabetes in animal models has impaired testicular function and decreased male fertility[71]. The diabetogens enhance generation of reactive oxygen species[72], consequently, it induces both lipid peroxidation and protein carbonyl production in the testes[20,73]. Additionally, the oxidative damage associated with the DM is found to be associated with genotoxic consequences[20,74]. In this study, we revealed that the treatment of GA decreased MDA concentrations of diabetic rats. Also, the treatment of GA increased the activities of CAT, SOD, and GPx in the testis of diabetic rat. Our finding are agree with earlier reports that insoluble dietary fiber from wheat bran (contains some feruloyl groups) increased GPx, and SOD activities while decreased MDA levels in the testis of diabetic rat[75]. In addition, ginger, a dietary fibre was found to ameliorate the SOD, CAT and GPx activities testis of diabetic rat[59]. Moreover, sperm count, percentages of sperm motility and viability increased in diabetic rats treated with a combination of ginger and cinnamon[76].
Increased oxidative stress together with failure of antioxidant defense system is found to be the fundamental factors leading to the diabetic pathogenesis and its complications. In this study, quantitative PCR used to investigate whether steady-state transcription abundant was altered. The treatment of GA significantly increased testicular mRNA expression of GPx and SOD. However, no changes were observed in CAT mRNA expression in all groups regardless of GA treatment. The increases in GPx and SOD mRNA corresponding with increases in their activities suggest the role of post-translational modification in altering the activities of these enzymes[77]. Yet, the disparity between the activity of CAT and its mRNA expression in this study may point out the presence of very multipart mechanisms regulating the activity of CAT to protect the oxidative damage[78]. Moreover, the histological features of the testis in showed marked degeneration in the testis of alloxan-induced diabetic rats. But the treatment of GAsignificantlyprotected the testis of diabetic rats from oxidative damage.
We conclude that the treatment of GA improved semen quality, reduced lipid peroxidation, improved the activities of testicular antioxidant enzymes activities together with their mRNA expression. In addition, the treatment of GA decreased testicular damage. Thus, GA may be of useful in the reduction of oxidative stress and improvement of reproductive performance in diabetic patient.
Conflict of interest statement
The authors declare that they have no competing interest.
Acknowledgments
The authors are highly grateful to Professor Zhao Ruqian and Shi-Xiong Shi Professor atNanjing Agricultural University. Thanks are also conducted to Professor Yue-Pu Pu at Southeast University , China for their support to conduct this research
[1] Ji HF, Li XJ, Zhang HY. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia?EMBO Rep2009;10: 194-200.
[2] Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads.Biochim Biophys Acta2013;1830(6): 3670-3695.
[3] Watal G, Dhar P, Srivastava SK, Sharma B. Herbal medicine as an alternative medicine for treating diabetes: the global burden.Evid Based Complement Alternat Med2014;2014: 596071.
[4] Rutebemberwa E, Lubega M, Katureebe SK, Oundo A, Kiweewa F, Mukanga D. Use of traditional medicine for the treatment of diabetes in Eastern Uganda: a qualitative exploration of reasons for choice.BMC Int Health Hum Rights2013;13: 1-1.
[5] Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: A Systematic Analysis of 277 Health Surveys.PLoS Med2012;9(12): e1001356.
[6] Hadjkacem Loukil L, Hadjkacem H, Bahloul A, Ayadi H. Relation between male obesity and male infertility in a Tunisian population.Andrologia2015;47: 282-285.
[7] Butler MG, McGuire A, Manzardo AM. Clinically relevant known and candidate genes for obesity and their overlap with human infertility and reproduction.J Assist Reprod Genet2015;32: 495-508.
[8] Begum BN, Hasan S. Psychological problems among women with infertility problem: a comparative study.J Pak Med Assoc2014;64: 1287-1291.
[9] Unuane D, Tournaye H, Velkeniers B, Poppe K. Endocrine disorders & female infertility, best practice & research.Clin Endocrinol Meta2011;25: 861-873.
[10] Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility.Reprod Biomed Online2014;28: 684-703.
[11] Agarwal A, Allamaneni SS. Free radicals and male reproduction. J IndianMed Assoc2011;109: 184-187.
[12] Wilhelm J, Vytasek R, Uhlik J, Vajner L. Oxidative stress in the developing rat brain due to production of reactive oxygen and nitrogen species, oxidative medicine and cellular longevity.Oxidative Med CellularLong2016;2016: 5057610.
[13] Venturini D, Simao AN, Barbosa DS, Lavado EL, Narciso VE, Dichi I, et al. Increased oxidative stress, decreased total antioxidant capacity, and iron overload in untreated patients with chronic hepatitis C.Dig Dis Sci2010;55: 1120-1127.
[14] Palafox-Carlos H, Ayala-Zavala JF, González-Aguilar GA. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants.J Food Sci2011;76: R6-R15.
[15] Canivell S, Gomis R. Diagnosis and classification of autoimmune diabetes mellitus.Autoimmun Rev2014;13: 403-407.
[16] Cooper ME, Perkovic V, McGill JB, Groop PH, Wanner C, Rosenstock J, et al. Kidney disease end points in a pooled analysis of individual patientlevel data from a large clinical trials program of the dipeptidyl peptidase 4 inhibitor linagliptin in type 2 diabetes.Am J Kidney Dis2015;66(3): 441-449.
[17] Adjemian MK, Volpe RJ, Adjemian J. Relationships between diet, alcohol preference, and heart disease and type 2 diabetes among Americans.PLoS One2015;10: e0124351.
[18] Beckman JA, Paneni F, Cosentino F, Creager MA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II.Eur Heart J2013;34(2): 2444-2452.
[19] Chandrashekar KN, Muralidhara. Evidence of oxidative stress and mitochondrial dysfunctions in the testis of prepubertal diabetic rats.Int J Impot Res2009;21: 198-206.
[20] Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Adv Exp Med Biol 2008; 636: 154-171.
[21] Dymkowska D, Drabarek B, Podszywalow-Bartnicka P, Szczepanowska J, Zablocki K, Hyperglycaemia modifies energy metabolism and reactive oxygen species formation in endothelial cellsin vitro.Arch Biochem Biophys2014;542: 7-13.
[22] Giacco F, Brownlee M. Oxidative stress and diabetic complications.Circ Res2010;107: 1058-1070.
[23] Brownlee M. The pathobiology of diabetic complications: A unifying mechanism.Diabetes2005;54: 1615-1625.
[24] Nowsheen S, Yang ES. Theintersectionbetween DNA damageresponsean dcelldeathpathways.Exp Oncol2012;34: 243-254.
[25]Matough F, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications.Sultan Qaboos Univ Med J2012;12: 5-18.
[26] Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species.Curr Diabetes Rev2008;4: 46-54.
[27] Jain GC, Jangir RN. Modulation of diabetes-mellitus-induced male reproductive dysfunctions in experimental animal models with medicinal plants.Pharmacogn Rev2014;8: 113-121.
[28] La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero AE. Diabetes mellitus and sperm parameters.J Androl2012;33: 145-153.
[29] Kumar P, Rao GN, Pal BB, Pal A. Hyperglycemia-induced oxidative stress induces apoptosis by inhibiting PI3-kinase/Akt and ERK1/2 MAPK mediated signaling pathway causing downregulation of 8-oxoGDNA glycosylase levels in glial cells.Int J Biochem Cell Biol2014;53: 302-319.
[30] Peng C, Ma J, Gao X, Tian P, Li W, Zhang L. High glucose induced oxidative stress and apoptosis in cardiac microvascular endothelial cells are regulated by FoxO3a.PloS One2013;8: e79739.
[31] Chakraborty PP, Ray S, Bhattacharjee R, Ghosh S, Mukhopadhyay P, Mukhopadhyay S, et al. Diabetes and primary infertility in young males: do not forget cystic fibrosis.Clin Diabetes2015;33: 80-83.
[32] Alves MG, Martins AD, Rato L, Moreira PI, Socorro S, Oliveira PF. Molecular mechanisms beyond glucose transport in diabetes-related male infertility.Biochim Biophys Acta2013;1832(5): 626-635.
[33] Sumpio BE. Contemporary evaluation and management of the diabetic foot.Scientifica2012;2012: 17.
[34] Rato L, Alves MG, Dias TR, Cavaco JE, Oliveira PF. Testicular metabolic reprogramming in neonatal streptozotocin-induced type 2 diabetic rats impairs glycolytic flux and promotes glycogen synthesis.J Diabetes Res2015;2015: 13.
[35] Liu J, Wang Y, Gong L, Sun C. Oxidation of glyceraldehyde-3-phosphate dehydrogenase decreases sperm motility in diabetes mellitus.Biochem Biophys Res Commun2015;465: 245-248.
[36] Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, et al. Insulin dependant diabetes mellitus: implications for male reproductive function.Hum Reprod2007;22: 1871-1877.
[37] Bartak V, Josifko M, Horackova M. Juvenile diabetes and human sperm quality.Int J Fertil1975;20: 30-32.
[38] Murray FT, Cameron DF, Orth JM, Katovich MJ. Gonadal dysfunction in the spontaneously diabetic BB rat: alterations of testes morphology, serum testosterone and LH.Horm Metab Res1985;17: 495-501.
[39] Ballester J, Munoz MC, Domingue J, Rigau T, Guinovart JJ, Rodriguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH-and LH-linked mechanisms.J Androl2004;25: 706-719.
[40] Al Hayek AA, Khader YS, Jafal S, Khawaja N, Robert AA, Ajlouni K. Prevalence of low testosterone levels in men with type 2 diabetes mellitus: a cross-sectional study.J Family Community Med2013;20: 179-186.
[41] Ali BH, Ziada A, Blunden G. Biological effects of gum arabic: a review of some recent research.Food Chem Toxicol: Int J Brit Industrial Biol Res Assoc2009;47:1-8.
[42] Tyler VB, Robbers L, Robbers JE.Pharmacognosy. 8th ed. Philadelphia: Lea & Febiger; 1977, p. 64-68.
[43] Al-Majed AA, Mostafa AM, Al-Rikabi AC, Al-Shabanah OA. Protective effects of oral arabic gum administration on gentamicin-induced nephrotoxicity in rats.Pharmacol Res2002;46: 445-451.
[44] Al-Majed AA, Abd-Allah AR, Al-Rikabi AC, Al-Shabanah OA, Mostafa AM. Effect of oral administration of Arabic gum on cisplatin-induced nephrotoxicity in rats.J Biochem Mol Toxicol2003;17: 146-153.
[45] Abd-Allah AR, Al-Majed AA, Mostafa AM, Al-Shabanah OA, Din AG, Nagi MN. Protective effect of arabic gum against cardiotoxicity induced by doxorubicin in mice: a possible mechanism of protection.J Biochem Mol Toxicol2002;16: 254-259.
[46] Ali BH, Al-Husseni I, Beegam S, Al-Shukaili A, Nemmar A, Schierling S, et al. Effect of gum arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats.PloS One2013;8: e55242.
[47] Nasir O, Umbach AT, Rexhepaj R, Ackermann TF, Bhandaru M, Ebrahim A, et al. Effects of gum arabic (Acacia senegal) on renal function in diabetic mice.Kidney Blood Press Res2012;35: 365-372.
[48] Nasir O. Renal and extrarenal effects of gum arabic (Acacia senegal)--what can be learned from animal experiments?Kidney Blood Press Res2013;37: 269-279.
[49] Ahmed AA, Fedail JS, Musa HH, Kamboh AA, Sifaldin AZ, Musa TH. Gum Arabic extracts protect against hepatic oxidative stress in alloxan induced diabetes in rats.Pathophysiology2015;22: 189-194.
[50] Adeyi AO, Idowu BA, Mafiana CF, Oluwalana SA, Ajayi OL, Akinloye OA. Rat model of food-induced non-obese-type 2 diabetes mellitus: comparative pathophysiology and histopathology.Int J Physiol Pathophysiol Pharmacol2012;4: 51-58.
[51] Bloom RJ, Westerfeld WW. The thiobarbituric acid reaction in relation to fatty livers.Arch Biochem Biophys1971;145: 669-675.
[52] Minami M, Yoshikawa H. A simplified assay method of superoxide dismutase activity for clinical use.Clin Chim Acta1979;92: 337-342.
[53] Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts.Anal Biochem1970;34: 30-38.
[54] Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase.J Lab Clin Med1967;70: 158-169.
[55] Musa HH, Ahmed AA, Musa TH, Fedail JS. Gum arabic down-regulate PPAR-γ and SCD mRNA expression in mice.Polish Ann Med2015;22(1): 11-17.
[56] Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.Methods2001;25: 402-408.
[57] Gado AM, Aldahmash BA. Antioxidant effect of Arabic gum against mercuric chloride-induced nephrotoxicity.Drug Design Dev Ther2013;7: 1245-1252.
[58] Ahmed AA, Fedail JS, Musa HH, Musa TH, Sifaldin AZ. Gum Arabic supplementation improved antioxidant status and alters expression of oxidative stress gene in ovary of mice fed high fat diet.Middle East Fertil Soc J2016;21: 101-108.
[59] Ghlissi Z, Atheymen R, Boujbiha MA, Sahnoun Z, Makni Ayedi F, Zeghal K, et al. Antioxidant and androgenic effects of dietary ginger on reproductive function of male diabetic rats.Int J Food Sci Nutr2013;64: 974-978.
[60] Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health.Nutrients2010;2: 1266-1289.
[61] Talati R, Baker WL, Pabilonia MS, White CM, Coleman CI. The effects of barley-derived soluble fiber on serum lipids.Ann Fam Med2009;7: 157-163.
[62] Cho SH, Kim TH, Lee NH, Son HS, Cho IJ, Ha TY. Effects of Cassia tora fiber supplement on serum lipids in Korean diabetic patients.J Med Food2005;8: 311-318.
[63] Diez R, Garcia JJ, Diez MJ, Sierra M, Sahagun AM, Calle AP, et al. Hypoglycemic and hypolipidemic potential of a high fiber diet in healthy versus diabetic rabbits.Biomed Res Int2013;2013: 960568.
[64] Kelley JJ, Tsai AC. Effect of pectin, gum arabic and agar on cholesterol absorption, synthesis, and turnover in rats.J Nutr 1978;108: 630-639.
[65] Moundras C, Behr SR, Demigne C, Mazur A, Remesy C. Fermentable polysaccharides that enhance fecal bile acid excretion lower plasma cholesterol and apolipoprotein E-rich HDL in rats.J Nutr1994;124: 2179-2188.
[66] Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome.J Nutr Biochem2008;19: 71-84.
[67] Gallaher DD, Hassel CA, Lee KJ. Relationships between viscosity of hydroxypropyl methylcellulose and plasma cholesterol in hamsters.J Nutr1993;123: 1732-1738.
[68] Evans AJ, Hood RL, Oakenfull DG, Sidhu GS. Relationship between structure and function of dietary fibre: a comparative study of the effects of three galactomannans on cholesterol metabolism in the rat.Br J Nutr1992;68: 217-229.
[69] Eastwood MA. The physiological effect of dietary fiber: an update. Annu Rev Nutr 1992; 12: 19-35.
[70] Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review.J Food Sci Technol2014;51: 1021-1040.
[71] Navarro-Casado L, Juncos-Tobarra MA, Chafer-Rudilla M, de Onzono LI, Blazquez-Cabrera JA, Miralles-Garcia JM. Effect of experimental diabetes and STZ on male fertility capacity Study in rats.J androl2010;31: 584-592.
[72] Towler DA. Mitochondrial ROS deficiency and diabetic complications: AMP[K]-lifying the adaptation to hyperglycemia.J Clin Invest2013;123: 4573-4576.
[73] Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review.J Biochem Mol Toxicol2003;17: 24-38.
[74] Shrilatha B, Muralidhara. Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: its progression and genotoxic consequences.Reprod Toxicol2007;23: 578-587.
[75] Ou SY, Jackson GM, Jiao X, Chen J, Wu JZ, Huang XS. Protection against oxidative stress in diabetic rats by wheat bran feruloyl oligosaccharides.J Agric Food Chem2007;55: 3191-3195.
[76] Khaki A, Khaki AA, Hajhosseini L, Golzar FS, Ainehchi N. The antioxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr J Tradit Complement Altern Med 2014;11: 1-8.
[77] Limaye PV, Raghuram N, Sivakami S. Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocininduced diabetic rats.Mol Cell Biochem2003;243: 147-152.
[78] Sadi G, Guray T. Gene expressions of Mn-SOD and GPx-1 in streptozotocin-induced diabetes: effect of antioxidants.Mol Cell Biochem2009;327: 127-134.
[79] Gargari BP, Behzad MH, Ghassabpour S, Ayat A. Prevalence of overweight and obesity among high-school girls in Tabriz, Iran, in 2001.Food Nutr Bull2004;25: 288-291.
ment heading
10.1016/j.apjr.2016.07.014
*Corresponding author: Abdelkareem Abdallah Ahmed, Department of Physiology and Biochemistry, Faculty of Veterinary Science and Department of Biology, Faculty of Education, University of Nyala, Nyala, P.O Box: 155 Nyala, Sudan.
Fax: +249711833123
E-mail: jfedail2@gmail.com, kareemo151@gmail.com
 Asian Pacific Journal of Reproduction2016年5期
Asian Pacific Journal of Reproduction2016年5期
- Asian Pacific Journal of Reproduction的其它文章
- Freezability of buffalo semen with TRIS extender enriched with disaccharides (trehalose or sucrose) and different glycerol concentrations
- Profile of peroxidative injury and antioxidant indicators in singleton, twins and multiple bearing goats throughout pregnancy
- Conception rate in Holstein dairy cows having both normal sized follicles and cystic follicles at estrus
- Polymyxin B changes the plasma membrane integrity of cryopreserved bull semen
- Comparative blood and seminal plasma oxidant/antioxidant status of Arab stallions with different ages and their relation to semen quality
- Ovarian hyperstimulation syndrome followed by ovarian torsion in premenopausal patient using adjuvant tamoxifen treatment for breast cancer
