Profile of peroxidative injury and antioxidant indicators in singleton, twins and multiple bearing goats throughout pregnancy
Abdel-Ghani MA, T.M. El-sherry TM, Hayder M, Abou-Khalil NS
1Department of Theriogenology, Faculty of Veterinary Medicine, Assuit University, Assuit, 71526, Egypt
2Animal Production Research Institute, Agriculture Research Center, Dokki, Giza,12311, Egypt
3Department of Medical Physiology, Faculty of Medicine, Assiut University, Assuit, 71526, Egypt
4Laboratory of Theriogenology, Department of Veterinary Clinical Science, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo 060-0818, Japan
Profile of peroxidative injury and antioxidant indicators in singleton, twins and multiple bearing goats throughout pregnancy
Abdel-Ghani MA1,4*, T.M. El-sherry TM1, Hayder M2, Abou-Khalil NS3
1Department of Theriogenology, Faculty of Veterinary Medicine, Assuit University, Assuit, 71526, Egypt
2Animal Production Research Institute, Agriculture Research Center, Dokki, Giza,12311, Egypt
3Department of Medical Physiology, Faculty of Medicine, Assiut University, Assuit, 71526, Egypt
4Laboratory of Theriogenology, Department of Veterinary Clinical Science, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo 060-0818, Japan
ARTICLE INFO
Article history:
Received
Received in revised form
Accepted
Available online
Goats
Oxidative stress
Pregnancy
Reactive oxygen species
Objective:To investigate the changes in profile of the oxidant and antioxidant indicators throughout pregnancy in goats.Methods: Estrus in goats was synchronized using an intravaginal progestogen impregnated sponge and the buck was introduced in the herd during the experiment for breeding purpose. Serum nitric oxide (NO) as standard angiogenic marker, total antioxidant capacity (TAC) as a cryoprotectant indicator, total peroxide (TPX) as a pathogenetic effector were measured, followed by calculation of OSI% (TPX/TAC) ×100 as a reflector of the oxidant/antioxidant status, andmalondialdhyde were estimated.Results:Compared with values in singleton and twins bearing goats, TAC values in multiple bearing goats were higher in 4thmonth and lower in 5th month (P<0.05). Values of TPX were lower in multiple bearing goats in 4th month and higher in 5th month (P<0.05) when compared with singleton and twins bearing goats.In contrary, NO values started to increase from the 2nd month until 4th month of gestation in all pregnant goats. However, the NO was lower in 5th month (P<0.05) in multiple bearing goats.Values of NO were negatively correlated with OSI % in allbearing goats.Conclusion:Knowing the relationship between the fetal number and oxidative stress indicators could be useful in the clinical management of such pregnancies and could be useful in the early detection or prediction adverse pregnancy outcome. Particularly, the 4th and 5th month of gestation increases the liability to reactive oxygen species in goats.
1. Introduction
Reactive oxygen species (ROS) are normal products of aerobic metabolism that are called free radicals[1]. Oxidative stress occurs due to an imbalance between the ROS and the antioxidant level[2]. Pregnancy is a physiological period during which different metabolic pathways are altered, resulting in greater oxygen consumption, modification of energy substrates consumption, and high metabolic placental demands, with consequent increasing ROS and increasing oxidative stress[3,4]. Moreover, carrying multiple fetuses in ewes and does is implicated as a major risk factor in pregnancy toxemia that is strongly associated with the oxidative stress[5]. Oxidative stress resulted in macromolecule damage including lipid peroxidation, protein crosslinking, DNA damage, and changes in the growth and function of cells[6]. Control mechanisms of oxidative damage during caprine gestation offer great opportunity for antioxidants supply to developing fetuses, in preparation for extrauterine life, giving them a major shield against free radicals loaded environments. One expanding area of research not well elucidated to date is oxidant/ antioxidant status during gestational stages in different animal species. Although there is extensive data for humans, no reports about the mechanisms of protection during pregnancy are available to date for the caprine species. Some studies have reported increased oxidative stress and altered status of antioxidant enzymes in specific situations, such as copper-ascorbate induced toxic injury[7] andintravaginal sponge application[8].
As a result of limited availability of specific oxidative stress biomarkers[9], measurement of oxidative stress index (OSI) which estimated from ratio of total peroxide (total peroxide (TPX); pathogenetic effector) to total antioxidant capacity (total antioxidant capacity (TAC); cytoprotectant indicator) is a widely used a reflector of the whole oxidant/antioxidant landmarks[7,10].
During pregnancy, nitric oxide (NO) is an important regulator of blood flow contributing to maternal systemic vasodilatation (potent vasodilator agent), regulates uterine and feto-placental blood flow[11]. Furthermore, it is an endothelial survival factor, inhibiting apoptosis and enhancing endothelial cell proliferation[12], hence, it is considered as a gold standard vasodilator and angiogenic marker.
In the view of these considerations, the objective of the current study was to investigate the changes in profile of oxidative stress indicators in single, twins and multiple bearing does.
2. Materials and methods
2.1. Animals
Adult female goats (n=50; aged as 1.5-2.5 years; weighting 20–25 kg) were selected from the lot maintained at Mallawi Animal Production Research Station, El-Minia, Egypt (latitude 28°07´N and 30°33´E) during Autumn. Sa'idi goats are a local Egyptian breed found in the southern part of the country. The animals were kept mainly for meat production. Goats were maintained graze on in pens during all the day. Water and a mineral supplement were available ad libitum. The management of the goats did not change throughout the experimental period. Estrus in goats was synchronized using an intravaginal progestagen impregnated sponge (40 mg fluorogestone acetate, GFA, Chronogest®, Intervet, International,boxmeer, Netherland) for 5 d, and were injected with 2.5 mg of dinoprost (Lutalyse, Pfizer manufacturing, Purts, Belgium) at the time of sponge insertion. At the time of sponge removal, 10.5 µg busereline acetate (GnRH, Receptal, Intervet) i.m. were injected. Goats were considered to be in estrus only if they stood while being mounted by the bucks. Bucks were introduced in the herd during the experiment for breeding purpose. Mating was scheduled every 4 h until does refuse to be mounted by bucks. The mated goats were recorded and kept under close observation until parturition.
2.2. Pregnancy diagnosis and ultrasound scanning
Ultrasound scanning was performed by the same operator between 09.00 a.m. and 01.00 p.m. using an ultrasound scanner (Mylab 30, Piemedical, Netherlands), equipped with a 6–8 MHz endorectal linear probe (Lv513). On day 30 after insemination, the does were checked for pregnancy using the B-mode transrectalultrasonography method.Thetransrectal ultrasonography was performed every 30 d during the overage of the pregnancy.
Each doe was restrained in a standing position and the abdominal wall was compressed to facilitate the visualization of the uterus. The rectum was lubricated using hydrosoluble contact gel prior to insertion of the transducer; the probe was positioned perpendicularly to the abdominal wall and the bladder was identified to orientate the visualization of the uterine horns. The transducer was rotated 90 clockwise and 180 anticlockwise in order to orient and image the entire reproductive tract. A total of 44 does were pregnant (singleton bearing goats,n=12; twins bearing goatsn=14; multiple bearing goats,n=18). The numbers of foeti were confirmed after parturition and the aborted foeti were excluded from counting in this study.
2.3. Blood sample collection
Blood samples were collected via jugular venipuncture every month just after each ultrasound scanning.Whole blood was drawn into vacutainer tube containing no anticoagulant. Then, it was incubated in an upright position at room temperature for 30 min to clot before spinning and separating, and centrifuged for 10 min at 1000g. Supernatant (serum) was aspirated at RT and pool into a cryovials and stored at –80 ℃ until use.
2.4. TAC assay
Serum TAC was evaluated according to protocol given by Koracevicet al.[13] . Briefly, 0.02 mL of distilled water was added in blank tube to 0.5 mL of R1 (H2O2diluted 1000 times before use), whereas 0.02 mL of sample was added in sample tube. Then, the tubes were mixed and incubated 10 min at 37 ℃. Working reagent was prepared by mixing equal volumes of R2(chromogen) and R3(enzyme and buffer) immediately before use, and then 0.5 mL of the working reagent was added to both of blank and sample tubes. All tubes were mixed and incubated 5 min at 37 ℃. The absorbance of blank (ABlank) and sample (ASample) were read immediately against distilled water at 505 nm. TAC levels in the samples were calculated based on the following equation:
Serum TAC level (mM/L) = ABlank- ASample×3.33
2.5. TPX assay
Serum TPX was assessed following Harmaet al.[14]. Briefly, 50 mL serum was added to 450 µL the reagent and blank reagent in separate Eppindorff's tubes (T8911, Eppendorf®Safe-Lock microcentrifuge tubes, volume 0.5 mL Sigma, St. Louis, MO, USA), mixed well and incubated at room temperature for 30 min, centrifuged at 5000 g for 3 min, and 250 µL of the supernatant was transferred into wells of 96-well microplate and optical density of the formed purple chromophore was recorded at 560 nm against reagent blank-treated samples. Standards were treated similarly without a blank reagent treatment. Concentration of TP in samples was calculated from the standard curve constructed using standard concentrations and readings after subtracting the reagent blank readings for samples.
2.6. OSI calculation
It considered as reflector of the oxidant/antioxidant status. It is the percent ratio of TPX content to TAC concentration[14], and measured according to the following equation:
Serum OSI = TPX (µM/L) / TAC (µM/L)×100
2.7. NO assay
Serum NO was measured according to Montgomery and Dymock[15]. Briefly, 0.1 mL of serum and 1 mL of R2 were added into both of sample and sample blank tubes; while, 0.1 of reagent 1 R1and 1 mL of R2were pipetted into tubes labeled as standard and standard blank. All the tubes were mixed and allowed to stand for 5 min, followed by addition of 0.1 mL of R3into sample and standard tubes. After well mixing, all the tubes were allowed to stand for another 5 min. Then absorbance of sample (ASample) against sample blank, and of standard (AStandard) against standard blank were read at 540 nm, and the value of NO was obtained using the followingequation:

2.8. Malondialdhyde (MDA) assay
Serum MDA was measured according to Ohkawaet al.[16] procedure. Briefly, 1 mL of chromogen was pipetted into tubes labeled as sample, standard, and blank. Then, 0.2 mL of sample and standard were added to the corresponding tubes. All the tubes were mixed, covered with screw cap, and then heated in boiling water bath for 30 min. After cooling the mixture, 0.2 mL of sample was added to blank tube and mixed well. The absorbance of the resultant pink product against blank, and standard against distilled water were read at 534 nm, and the MDA concentrations in the samples were recorded depending on the following equation:
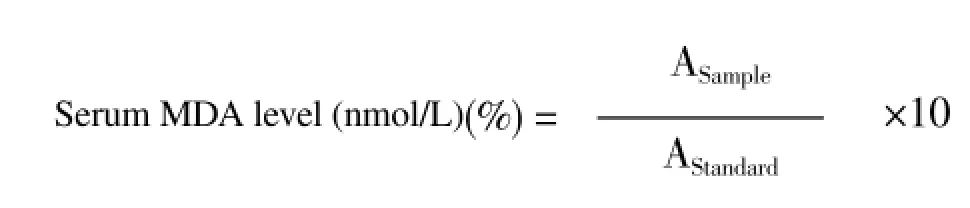
2.9. Statistical analysis
Plasma levels of TAC, TPX and NO were analyzed by repeated measure with time using analysis of variance (ANOVA) to determine main effects of group or group by month. When main effect of group or group by month was observed, the differences of group means at specific time point were analyzed by the Student’s t-test using JMP statistical software (version 5.1; SAS Institute, Cary, NC, USA). Graphpad Prism v5 software (Graphpad Software, Inc., San Diego, CA) was used to draw the figures. The differentcewere significant atP<0.05. The data were expressed as mean ± SD.
All experiments were carried out in accordance with the guidelines for the care and use of the animals approved by Veterinary Teaching Hospital's Animal Care Committee, Assuit University.
3. Results
The results of TAC, TPX, NO, MDA and OSI mean values were delineated in Figure 1. From 1st to 5th month, results showed that there was a difference between groups (P<0.001) and group by month (P<0.001). In singleton bearing goats, the TAC values were similar (P>0.05) throughout the pregnancy. Similarly, in twins bearing goats, there was no difference (P>0.05) in the TAC values. However, in multiple bearing goats, TAC values were higher (P<0.05) during the 3rd and 4th months of pregnancy. Compared with values in singleton and twins bearing goats, the mean TAC values in multiple bearing goats were higher in 4th month and lower in 5th month (P<0.05).
Serums TPX mean values decreased throughout gestation in all pregnancies, with the exception of 5th month. Values of TPX were lower in multiple bearing goats in 4th month and higher in 5th month (P<0.05) when compared with singleton and twins bearing goats. In contrary, mean NO values started to increase from the 2nd month and continued until 4th month of gestation in singleton, twins and multiple bearing goats. However, the NO values were lower during the 5th month (P<0.05) in multiple bearing goats.
Mean values of OSI% decreased starting from 2nd month of gestation in all groups, with the exception of 5th month in multiple bearing goats, whereas, mean values of OSI% were higher in 5th month (P<0.05) compared with singleton.
Values of NO was negatively correlated with OSI% in singleton, twins and multiple bearing goats (NOvs.OSI% in singleton,r=-0.65,P<0.0 001; NOvs.OSI% in twins,r=-0.72,P<0.0 001 and NOvs.OSI% in multiple,r=-0.87,P<0.0 001).
In singleton bearing goats, serum MDA level was increased (P<0.05) during pregnancy starting from 2nd month of pregnancy. In twins bearing goats, the MDA level was similar (P>0.05) during the 1st, 2nd, 3rd and 4th months of pregnancy, however, MDA level was higher (P<0.05) during the 5th month. In multiple bearing goats, there was no difference (P>0.05) during the first three months of pregnancy, however, it was higher (P<0.05) during the 4th and 5th months of pregnancy. Additionally, there was no difference (P>0.05) in MDA levels between three pregnant groups during the 1st month of pregnancy. Additionally, serum MDA level in pregnant goats bearing twins and multiple was higher (P<0.05) than that found in pregnant goats bearing singleton during the 2nd, 4th and 5th months of pregnancy. However, the MDA level was similar (P>0.05) in both pregnant goats bearing singleton and twins during the 3rd month of pregnancy.
4. Discussion
Placenta throughout pregnancy is a site of active oxygen metabolism that continuously generates oxidative stress. Oxidative stress are associated with adverse pregnancy outcomes including abortion, poor birth and restriction of fetal growth[3,4]. An arguable data has been available on the oxidative and antioxidative status during normal pregnancy. In the current study, absence of significant changes in serum TPX levels in goats carry singleton against those carrying twins along the whole length of gestation are in harmony with the findings in Akkaraman[17] and Chios ewes[18], but inconsistent with those in Awassi ewes[8]. However, a significant difference was found in multiple bearing goats when compared to the other two groups. This inconsistency in the results may be attributing to differences in breed or types of samples.
Significant reduction in serum TAC levels in the 5th month of pregnancy with multiple versus singleton and twins nearing goats is corresponding to reduced activity of enzymatic antioxidants in ewes bearing multiple fetuses and those suffer from pregnancy toxemia[19,20]. It is possible that increased ROS generation caused by hyperketonemia and hypoglycemia in pregnancy with multiple fetuses[19,21] consumed antioxidants resulting in a dramatic reduction in their levels.
Calculated from ratio of TPX to TAC, OSI is a promising comparative index representing the interaction between the radical generating factors and their counter-players, and was significantly higher in does carrying multiple versus than those carrying singleton in the last month of gestation. This finding is due to marked decrement in TAC pre-partum and revealing a greater chance of peroxidative injury in pregnant does with multiple fetuses.
Significant reduction in serum NO levels in the last month of prepartum is incompatible with elevation of its levels and metabolites in pregnancies with multiple versus singleton fetuses in one study[22] exemplifying a compensatory mechanism maintaining blood flow and fetal nutrient supply. However, the observed drop in NO in the current investigation can be explained by exhaustion of its level under devastating impact of oxidative stress, which illustrated by increased OSI in the last month of pregnancy in multiple fetusesopposed to single ones.
Serum OSI levels and NO were negatively correlated in pregnant does carrying single and multiple fetuses. In fact, oxidative stress decreased NO released by shunting it toward scavenging free radicals[23]. Lipid peroxidation are implicated in the development of pregnancy toxemia in ewes[19] with generation of free radicals especially superoxide anions which inhibit endothelial nitric oxide synthase protein expression[24,25], reacts with NO to form peroxynitrite[26] which in turn oxidizes the NOS cofactor tetrahydrobiopterin[27], and reduces cellular transport of L-arginine, the endothelial nitric oxide synthase substrate for NO production[28]. Negative correlation was found between serum OSI and blood flow volume in placentomes and was similar to ability of suramin, as an inducer of OS-derived placental dysfunction, to restrict maternal placental blood volume and increase interface between maternal and fetal circulation[29].
Positive correlation between serum NO levels on one side, and the time-averaged maximum velocity, and the blood flow volume on the other side in placentomes (data not shown her) is matched with a well-known key role of NO in attenuation of vascular reactivity of the uterine artery to vasoconstrictors during the course of pregnancy[30], and its ability to contributes to low systemic, umbilical, and uterine vascular resistance in the fetus[31]. These facts were confirmed by the observed negative correlation between NO production and placental vascular resistance with consequent compromised feto-placental and fetal systemic circulation[32].
Öztabaket al.[18] have been reported that, in Chios ewes, lipid peroxidation level during 4thand 5th months of pregnancy is not different from non-pregnant control. Similarly, Erisiret al.[8] have reported that no significant difference is observed between early and late pregnancy in Awassi ewes compared with non-pregnant ewes in MDA level. Güret al.[17] have reported that the MDA levels in ewes bearing twins were found higher than in both non-pregnant and pregnant with a singleton, and the increased MDA level in twins bearing ewes may be attributed to an increased oxygen requirement and increased circulating lipids. Our finding showed that there was no significant difference in MDA levels between three pregnant groups during the 1st month of pregnancy, however, the MDA levelin pregnant goats bearing twins and multiple was significantly higher than that found in pregnant goats bearing singleton during the 2nd, 4th and 5th months of pregnancy.
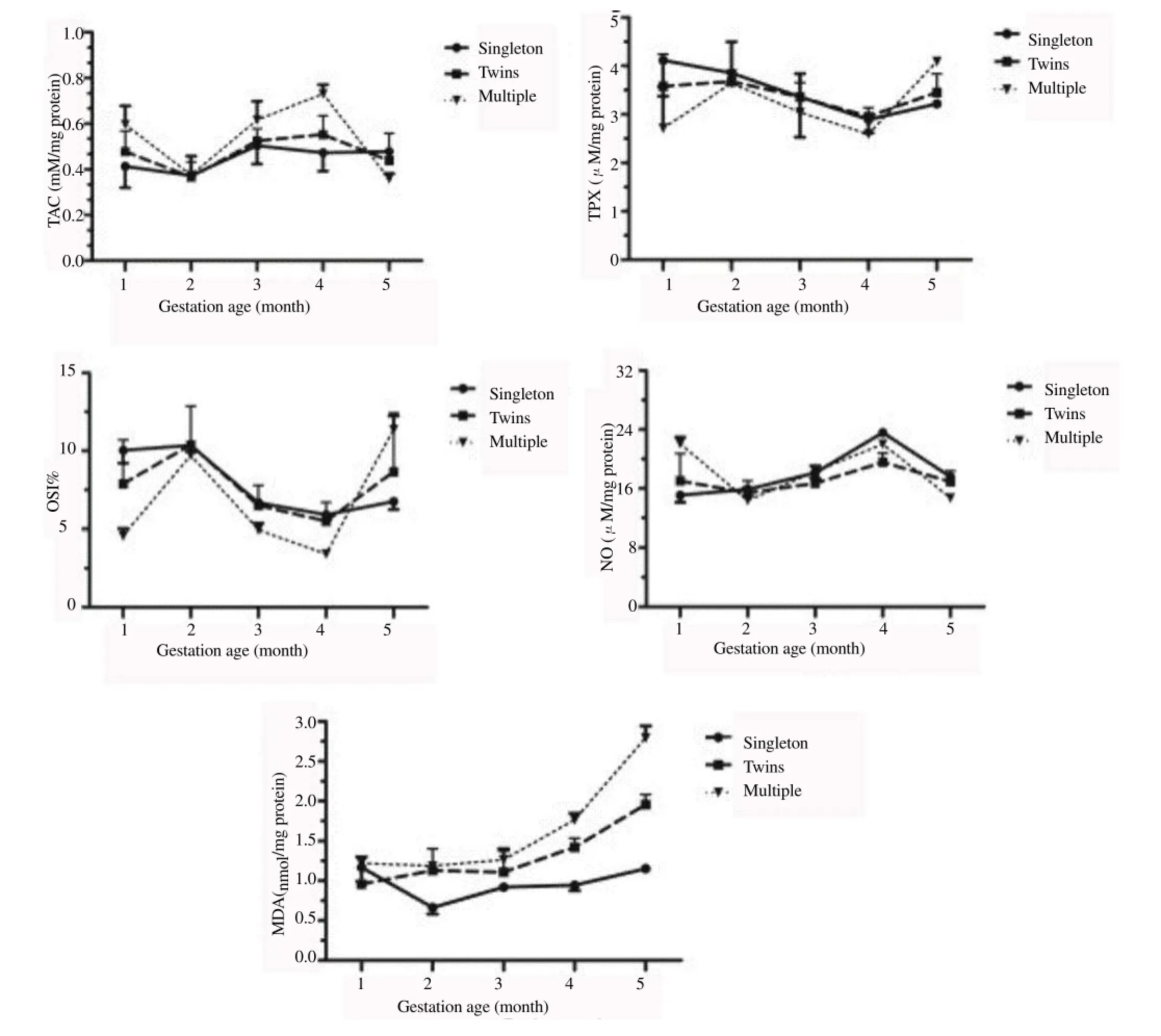
Figure 1.TAC, TP, OSI (total peroxide/total antioxidant capacity×100), NO and MDA in singleton, twins and multiple bearing goats.
Taking together, pregnant does bearing multiple fetuses suffered from oxidative stress burden as evident by reduction in TAC and elevation in OSI at the last month prepartum accompanied with marked decrement in NO levels at the last two months of gestation versus those bearing singletons. Dietary antioxidant supply in case of pregnancy with multiple fetuses is strongly warranted especially in the last month before parturition to avoid diminished vascular reactivity with detrimental consequences on fetal growth status.
In conclusion, the present study shows that the pregnancy with multiple foetuses in goats causes an increase in ROS and oxidative stress by increasing serum lipid peroxidation levels. Strikingly, the 4th and 5th month of gestation may increase the liability to ROS in goats. An understanding the relationship between the fetal number on one side and oxidative stress indicators on the other side could be useful in the clinical management of such pregnancies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
This study was kindly supported by project of Introducing Improved Genotypes and better managements to increase small ruminant roles in conventional Egyptian farm as an investment or income pattern. Grant No: 416 B.
[1] Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, et al.Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int 2014; 2014: 1-19.
[2] Gosmaro F, Bagnati M, Berto S, Bellomo G, Prenesti E. Measurement of total antioxidant capacity of human plasma: setting and validation of the CUPRAC-BCS method on routine apparatus ADVIA 2400. Talanta2013; 115: 526-532.
[3] Vannucchi CI, Jordao AA, Vannucchi H. Antioxidant compounds and oxidative stress in female dogs during pregnancy. Res Vet Sci2007; 83(2): 188-193.
[4] Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta2010; 31: Suppl: S66-S69.
[5] Olfati A, Moghaddam G, Bakhtiari M. Diagnosis, treatment and prevention of pregnancy toxemia in ewes. Int J Adv Biol Biom Res 2013; 1(6): 1452-1456.
[6] Ehsaei M, Khajavi M, Arjmand MH, Abuee MA, Ghayour-Mobarhan M, Hamidi Alamdari D. Prooxidant-antioxidant balance in patients with traumatic brain injury.Acta Neurol Belg2015; 115(1): 69-73.
[7] Chaudhary N, Parajuli A, Gurung B, Karki K, Rajbhandari P.Evaluation of oxidative stress and antioxidant status in iron deficiency anemia. Indian J Clin Biochem 2010; 25(4): 216-222.
[8] Erisir M, Benzer F, Kandemir FM. Changes in the rate of lipid peroxidation in plasma and selected blood antioxidants before and during pregnancy in ewes. Acta Vet Brno2009; 78(2): 237-242.
[9] Brock GR, Butterworth CJ, Matthews JB, Chapple IL. Local and systemic total antioxidant capacity in periodontitis and health. J Clin Periodontol 2004; 31(7): 515-521.
[10] Ibrahim HM. Oxidative stress associated with spasmodic, flatulent, and impaction colic in draft horses. J Equine Vet Sci 2014; 34: 1205-1210.
[11] Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol 1997; 272: R441-463.
[12] Moonmanee T, Navanukraw C, Uriyapongson S, Kraisoon A, Aiumlamaic S, Guntaprom S, et al. Relationships among vasculature, mitotic activity, and endothelial nitric oxide synthase (eNOS) in bovine antral follicles of the first follicular wave. Dome Anim Endocrinol 2013; 45:11-21.
[13] Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol2001; 54: 356-361.
[14] Harma M, Harma M, Erel O. Measurement of the total antioxidant response in preeclampsia with a novel automated method. Eur J Obstet Gynecol Reprod Biol 2005; 118(1): 47-51.
[15] Montgomery H, Dymock J 1961. Determination of nitrite in water. Inp. 414-&. Royal Soc Chemistry Thomas Graham House, Science Park, Milton RD, Cambridge CB4 0WF, Cambs, England.
[16] Ohkawa H, Ohishi N, Yagi K, Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem1979; 95: 351-358.
[17] Gür S, Türk G, Demirci E, Yüce A, Sönmez M, Ozer S, et al. Effect of pregnancy and foetal number on diameter of corpus luteum, maternal progesterone concentration and oxidant/antioxidant balance in ewes. Reprod Domest Anim2011; 46: 289-295.
[18] Öztabak K, Civelek S, Özpinar A, Burcak G, Esen F. The effects of energy restricted diet on the activities of plasma Cu-Zn SOD, GSH-Px, CAT and TBARS concentrations in late pregnant ewes. Turk J Vet Anim Sci2005; 29: 1067-1071.
[19] Al-Qudah KM. Oxidant and antioxidant profile of hyperketonemic ewes affected by pregnancy toxemia. Vet Clin Pathol 2011; 40: 60-65.
[20] Gurdogan F, Balıkçı E, Yıldız A. Some acute phase proteins, oxidative stress biomarkers and antioxidant enzyme activities in ewes with pregnancy toxemia. Iran J Vet Res 2014; 15: 297-299.
[21] Amador-Alvarado L, Montiel T, Massieu L. Differential production of reactive oxygen species in distinct brain regions of hypoglycemic mice. Metab Brain Dis 2014; 29: 711-719.
[22] Vonnahme KA, Wilson ME, Li Y, Rupnow HL, Phernetton TM, Ford SP, et al. Circulating levels of nitric oxide and vascular endothelial growth factor throughout ovine pregnancy. J Physiol2005; 565: 101-109.
[23] Kay HH, Grindle KM, Magness RR. Ethanol exposure induces oxidative stress and impairs nitric oxide availability in the human placental villi: a possible mechanism of toxicity. Am J Obstet Gynecol 2000; 182: 682-688.
[24] Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem 1998; 273: 24266 -24271.
[25] Brennan LA, Wedgwood S, Bekker JM, Black SM. Nitric oxide activates p21ras and leads to the inhibition of endothelial NO synthase by protein nitration. DNA Cell Biol 2003; 22: 317-328.μ
[26] Beckman JA, Goldfine AB, Gordon MB, Creager MA. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation 2001; 103: 1618 -1623.
[27] Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun1999; 263: 681-684.
[28] Ogonowski AA, Kaesemeyer WH, Jin L, Ganapathy V, Leibach FH, Caldwell RW. Effects of NO donors and synthase agonists on endothelial cell uptake of L -Arg and superoxide production. Am J Physiol Cell Physiol 2000; 278: C136 -C1343.
[29] Nash P, Eriksson UJ. Suramin-restricted blood volume in the placenta of normal and diabetic rats is normalized by vitamin E treatment. Placenta 2007; 28: 505-515.
[30] Xiao D, Liu Y, Pearce WJ, Zhang L. Endothelial nitric oxide release in isolated perfused ovine uterine arteries: effect of pregnancy. Eur J Pharmacol 1999; 367: 223-230.
[31] Yang D, Lang U, Greenberg SG, Myatt L, Clark KE. Elevation of nitrate levels in pregnant ewes and their fetuses. Am J Obstet Gynecol1996; 174: 573-577.
[32] Di Iorio R, Marinoni E, Coacci F, La Torre R, Cosmi EV. Amniotic fluid nitric oxide and uteroplacental blood flow in pregnancy complicated by intrauterine growth retardation. Br J Obstet Gynaecol1997; 104: 1134-1139.
ment heading
10.1016/j.apjr.2016.07.010
*Corresponding author: Abdel-Ghani MA, Department of Theriogenology, Faculty of Veterinary Medicine, Assuit University, Assuit, 71526, Egypt, Laboratory of Theriogenology, Department of Veterinary Clinical Science, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo 060-0818, Japan.
Tel: (+81)-011-706-5232
Fax: (+81)-011-706-5232
E-mail: Abdel-Ghani2016@outlook.com
Founding project:This study was supported by founding project of Introducing Improved Genotypes and better managements to increase small ruminant roles in conventional Egyptian farm (Grant No: 416 B).
 Asian Pacific Journal of Reproduction2016年5期
Asian Pacific Journal of Reproduction2016年5期
- Asian Pacific Journal of Reproduction的其它文章
- Heparin binding proteins and their relationship with vital sperm function tests visà-vis fertility of buffalo bull semen
- Conception rate in Holstein dairy cows having both normal sized follicles and cystic follicles at estrus
- Polymyxin B changes the plasma membrane integrity of cryopreserved bull semen
- Freezability of buffalo semen with TRIS extender enriched with disaccharides (trehalose or sucrose) and different glycerol concentrations
- Gum arabic improves semen quality and oxidative stress capacity in alloxan induced diabetes rats
- Ovarian hyperstimulation syndrome followed by ovarian torsion in premenopausal patient using adjuvant tamoxifen treatment for breast cancer
