Comparative blood and seminal plasma oxidant/antioxidant status of Arab stallions with different ages and their relation to semen quality
Gamal A. El Sisy, Amal M. Abo El-Maaty*, Zaher M. Rawash
1Department of Animal Reproduction and A.I., National Research Center, Dokki, Giza, Egypt
2Department of Artificial Insemination and Embryo Transfer, Animal Reproduction Research Institute, Agriculture Research Center, Giza, Egypt
Comparative blood and seminal plasma oxidant/antioxidant status of Arab stallions with different ages and their relation to semen quality
Gamal A. El Sisy1, Amal M. Abo El-Maaty1*, Zaher M. Rawash2
1Department of Animal Reproduction and A.I., National Research Center, Dokki, Giza, Egypt
2Department of Artificial Insemination and Embryo Transfer, Animal Reproduction Research Institute, Agriculture Research Center, Giza, Egypt
ARTICLE INFO
Article history:
Received
Received in revised form
Accepted
Available online
Arab stallion
Semen
Zinc
Copper
Ascorbic acid
Objective:To investigate the antioxidant/oxidant levels in blood serum and seminal plasma of Arab stallion with different ages and their relation to semen quality.Methods:Healthy Arabian stallions (n=57), were divided into three groups. Young (5-10 years), Moderate (11-16 years) and Old stallions (>16 years) were subjected to semen evaluation. Seminal plasma and blood samples were collected and stored at -20℃ for measuring glutathione reduced, nitric oxide, Malondialdehyde, ascorbic acid, copper and zinc.Results:Old stallions had significantly greater (P<0.05) ejaculate volume, % live sperm, and total sperm number compared to young and moderate aged groups. The moderate age horses had significantly the lowest (P<0.05) sperm concentration. Compared to young horses, serum zinc concentrations of moderate and old horses were significantly high (P<0.0 001), but NO concentrations were significantly (P<0.05) low. Seminal plasma zinc, ascorbic acid and nitric oxide concentrations were significantly (P<0.05 and 0.01) high in young stallion group. No significant correlations were observed between seminal zinc, copper, MDA and semen variables. Meanwhile, significant negative correlations were observed between seminal plasma ascorbic acid concentration and all semen variables except total sperm number and sperm abnormities %. Significant correlations were observed between reduced glutathione and both of sperm motility and % of live sperm. Nitric oxide concentrations correlated directly with individual sperm motility but adversely with total sperm number.Conclusion:Stallion age has significant effect on some semen variables, antioxidant/oxidant status of either blood serum or seminal plasma.
1. Introduction
The sperm cell is one of the aerobic organisms that require oxygen. Spermatozoa are rich in targets for oxidative attack and the major source of ROS within the sperm is mitochondria[1]. Depleting Adenosine tri-phosphate within mitochondria causes loss of sperm motility, viability and capacity for fertilization[2,3]. Defective or immature sperms and semen leukocytes produces more ROS and causes sperm dysfunction in men[2] and stallions[4,5]. Physiological low levels of ROS are required for the capacitation process in bulls[6], and normal sperm function in fertile men[2]. Hydrogen peroxide is responsible for the acrosome reaction[7]. Sperm DNA damage of infertile men resulted from high levels of sperm-derived ROS[4,8,9]. The presence of little cytoplasm within the head of spermatozoa makes them deficient in antioxidants and DNA-repair systems[2]. In stallion semen, ROS damage cells by changing lipids, proteins and DNA. It was found that peroxidative stress triggers the mitogen activated protein kinase cascade and the extracellular signal-regulated protein kinasephosphorylation[5]. Spermatozoa are potentially susceptible to peroxidative damage caused by excess ROS due to high amounts of polyunsaturated fatty acids in membrane phospholipids and to sparse cytoplasm. High levels of ROS might be detrimental to stallion sperm survival during storage[10]. Spermatozoa plasma membrane contained high polyunsaturated fatty acids[11], so the deleterious effects of lipidperoxidation on spermatozoa are loss of motility and fertilizing capacity[12]. The epididymis and seminal plasma possesses a complex antioxidant system aiming to prevent oxidative damages on sperm lipids, proteins and DNA[13]. The main enzymatic scavenger in the stallion cauda epididymal fluid and seminal plasma is catalase[14]. Although equine spermatozoa have limited GPx and SOD-like activity but equine seminal plasma contains high activity of SOD[15]. Fertile stallion’s seminal plasma contains lower concentration of ascorbic acid than infertile ones. From ascorbic acid antioxidant propertiesis to diminish lipid peroxidation[15,16], to regenerate other antioxidant molecules, such as B-carotene, vitamin E and reduced glutathione from their relevant radical species[18] and to preserve membrane integrity of cooled equine sperm[19]. During equine semen storage partial removal of seminal plasma is required to improve sperm survival[20]. Partial seminal plasma removal during semen processing preservation results in removal of oxidative stress scavengers and in turn subjecting spermatozoa to the damaging effect of excessive reactive oxygen species[21].
Higher copper levels in blood serum[22], seminal plasma and sperm are conjugated with infertility[23], poor sperm maturation, motility and fertility in boar[24], ram and bull[25], water buffaloes[26]. In stallions, seminal plasma zinc deficiency not only affects testes development and spermatogenesis[22,27] but also plays a role in protecting sperms during freezing[28].
This study aimed to compare blood serum and seminal plasma copper, zinc, reduced glutathione, ascorbic acidandnitric oxidein Arab stallion with different ages and their relationship to sperm quality.
2. Materials and methods
2.1. Semen collection, evaluation and semen analysis
A total of 57 healthy Arabian stallions, between 6 and 26 aged years were used during this study. These stallions belonged to Police Academy Stud (Cairo) , and several private horse studs (Giza) Egypt, and they have been used as sires in the regular natural breeding program of the stud. Stallions were divided according to age into young (5-10) years old; moderate (11-15) years old; and old (>15) years old. Three semen ejaculates were collected for each stallion at weekly interval. At the time of collection, early in the morning, a mare in estrus was used as a mount animal. Semen was collected using a lubricated and pre-warmed (45 to 50 ℃) Colorado model artificial vagina with an inline filter to separate the gel fraction. Immediately after collection, semen samples were transferred to a well-equipped laboratory and the gel-free portion of the ejaculate was evaluated for volume, progressive motility, and concentration was determined by conventional methods[29]. Spermatozoa motility was examined and recorded using a pre-warmed stage of microscope (200×). Sperm concentration was determined with a hemocytometer. Total sperm count per ejaculate was calculated from volume andsperm concentration. The pH was determined with teststrips (Merck, Darmstadt, Germany) and the morphological examination of semen samples was determined using nigrosineosin stain[30]. To obtain seminal plasma, an aliquot of semen was immediately centrifuged after semen collection at 1000×g for 10 min. The procedure was repeated with the supernatant of the first centrifugation to insure sperm free seminal plasma and stored at -20℃ until they were analyzed.
2.2. Blood sampling and analytical methods
Blood for was collected from the external jugular vein into vacuum tubes. Immediately after collection, the blood samples were centrifuged at 1000×g for 15 min, and sera was stored at -20 ℃until they were analyzed.
2.3. Blood antioxidant and biochemical analysis
Serum and seminal plasma glutathione (GSH) reduced[31], nitric oxide (NO)[32], lipid peroxide product (Malonedialdehyde, MDA) [33], Ascorbic acid[34], copper and zinc[35] were measured using commercial kits (Bio diagnostic, Egypt).
2.4. Statistical analysis
All data were analyzed using the SPSS statistical software[36]. The data were expressed as mean±SEM. Independent samplet-test, simple one way ANOVA and Pearson correlation test were used. Duncan Multiple Range test was used to differentiate between significant means atP<0.05.
3. Results
Seminal plasma (Table 1) contained significantly high concentrations of zinc (P<0.0001), NO (P<0.05), reduced glutathione (P<0.0 001), ascorbic acid (P<0.001) and MDA (P<0.05) but nearly similar copper compared to their serum levels. Semen characteristics of the three age groups (Table 2) showed a significant (P<0.05) increase of ejaculate volume and % of live sperms (P≤0.05), but tendency to a decrease of abnormal sperm % for horses older than 15 years (O). Stallions of moderate age had significantly low sperm cell concentrations (P<0.05) and total sperm count (P<0.01) compared to young and old stallions (Table 2).
Blood serum of old stallions had significantly (P<0.05) high zinc but significantly low NO (P<0.01), compared to the other age groups (Table 3). Moderate aged stallions had significantly low zinc (P=0.03), GHD and MDA (P<0.05). Seminal plasma of young stallions (Y, Table 4) had significantly the highest zinc (P<0.01), NO (P<0.0001) and ascorbic acid (P<0.01) concentrations compared to the other age groups and tended to havehigh MDA and GHD (P>0.05)
Seminal plasma zinc had only negative correlation with sperm individual motility (r=-0.50;P<0.05) and tended to correlate with total sperm number (r=0.39;P>0.05), but copper had no significant correlations with semen evaluation parameters (Table 5). Ascorbic acid had significant negative correlation with sperm individual motility (r=-0.69;P=0.02), live sperm % (r=-0.64;P=0.035) and sperm cell concentration (r=-0.67;P=0.02), but has a positive correlation with abnormal sperm % (r=0.54;P>0.05). Reduced glutathione (GHD) has strong correlation with sperm individual motility (r=0.81;P=0.005), live sperm % (r=0.64;P=0.03), and sperm cell concentrations (r=0.58;P=0.05). NO had a significant positive correlation with sperm individual motility (r=0.69;P=0.01), and live sperm % (r= 0.467;P=0.05), but negatively with total sperm number (r=-0.69;P<0.05), and semen volume (r=-0.68;P<0.05). MDA has positive correlation with total sperm number (r=0.64;P<0.05), semen volume (r= 0.58;P=0.05), but a negative correlation with abnormal sperm % (r=-0.74;P<0.05).
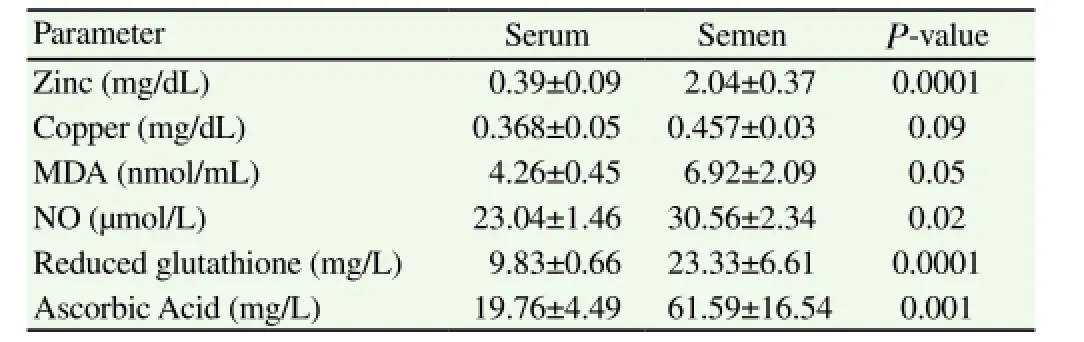
Table 1Mean ± SEM of blood serum and seminal plasma antioxidant status, zinc and copper of Arab stallions.

Table 2Mean ± SEM of semen parameters of young (5-10 years), moderate (11-15 years), and old (>15 years) Arab stallions.
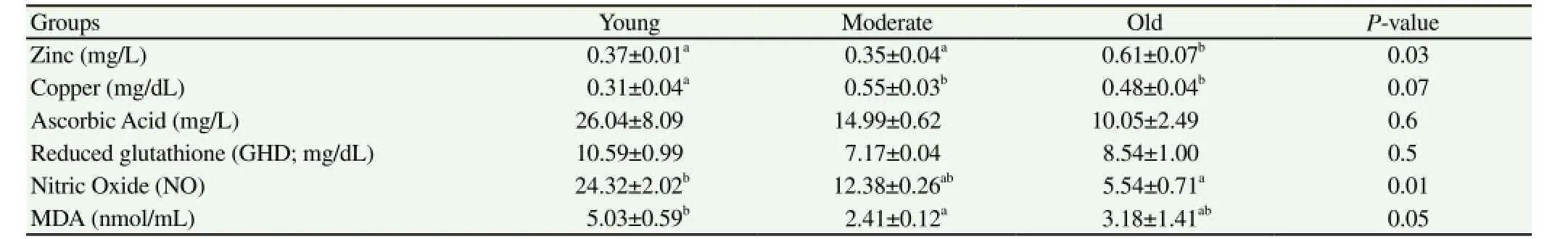
Table 3Mean ± SEM of blood serum antioxidant status, zinc and copper of Arab stallions.
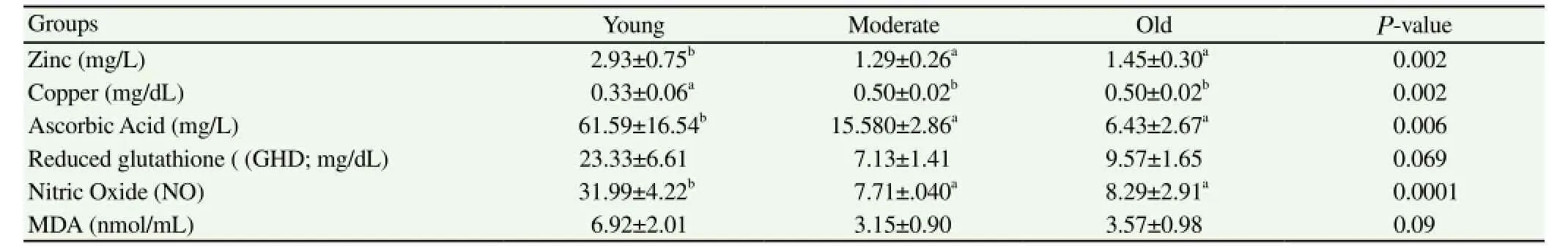
Table 4Mean ± SEM of seminal plasma Antioxidant status, zinc and copper of Arab stallions
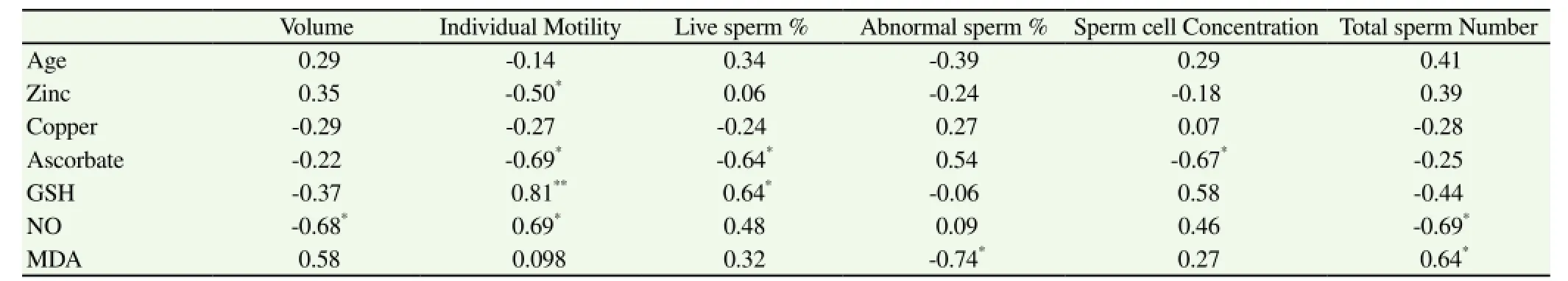
Table 5Correlations among seminal plasma antioxidant, zinc and copper concentration and seminal variables.
4. Discussion
Seminal plasma is an essential part of the ejaculate and it is involved in sperm functional activity, metabolism, survival and transport through the female reproductive tract. However, the deleterious action of equine seminal plasma on sperm motility is still controversial[37-39]. Moreover, a decrease of frozen–thawed semen motility was noticed when seminal plasma was not fully removed prior to cryopreservation[37]. Evaluating trace elements, antioxidant status and oxidative stress could be supportive to traditional semen evaluation in equine. In the current work, older stallions had significantly greater ejaculate volume, % live sperm, sperm concentration and total sperm number compared to the other two young groups, whereas the middle aged group showed lowest value for sperm concentration than younger and older groups. No significant age effect on mass and individual sperm motility, pH and total sperm abnormalities was observed. Similar to our results, age of Arab stallions had no effect on sperm motility and sperm concentration but affected total sperm abnormalities[40]. Similar to our findings, sperm abnormalities of aged Arab stallions were low compared to younger ones[40].
Zinc and copper in the body fluids are involved in cell protection against reactive oxygen species. Abnormal concentrations of seminal plasma zinc and copper are correlated with human infertility[41]. Copper exacerbate the damaging effects of ROS on sperm function[9]. The increase of copper concentration than normal values induces a toxic effect on spermatozoa by reducing oxidative processes and glucolysis that may reduce motility and viability[41]. Seminal plasma contained >6 folds higher zinc than blood serum and the improved semen parameters of old stallions not only refer to its higher level in blood serum but also in seminal plasma. Higher zinc concentrations were also reported in seminal plasma of stallions[28], and boar[24].As well as, blood serum zinc and seminal plasma zinc significantly related, and no correlation between blood serum zinc, copper concentration and the stallion’s semen quality were even reported[42]. Seminal plasma zinc concentration was not related with either sperm density or motility[43]. Although a non significant correlations between zinc and semen parameters except sperm individual motility were observed in stallions of this study but a significant positive correlation between zinc and semen volume and sperm concentration[44]. In rams, a significantly positive correlation between zinc and sperm motility percentage and sperm concentration[45].
Although seminal plasma copper levels of stallions were low[28], but significant individually variations in stallion seminal plasma copper concentration were also reported[28]. No correlation between copper levels of seminal plasma and semen variables in stallion[44]. However, serum copper was related to stallion semen characteristics[42]. Agedependent differences in the concentration of zinc and copper within the blood serum were found[42]. Concentrations of zinc and copper in blood and semen of stallion may be related to the individual stallions, variation in dietary regimen and environmental condition, more than its relations to stallion age. In rams, significant negative correlation between copper, sperm motility %, sperm concentration and spermatozoa abnormalities[45].
Seminal plasma ascorbic acid had been conjugated to males with history of sub-fertility or idiopathic infertility[46]. Nevertheless, lower ascorbic acid concentrations were observed in normozoospermic than normal men[47]. Moreover, addition of ascorbic acid to extender was beneficial for maintaining membrane integrity of cooled equine sperm[19].
In the current study, seminal plasma and blood serum ascorbic Acid concentrations were significantly higher in younger stallions and were decreasing with advancing age. In contrast higher ascorbic acid concentrations were observed in older stallions that increased with increasing age and adversely related to fertility[40]. In horses of the current study, seminal plasma content of ascorbic acid was 3 fold higher than serum levels, but in men seminal plasma concentration of ascorbic acid was 8–10–fold higher than blood[48]. Concentration of ascorbic acid in testes and seminal plasma is extremely sensitive to its blood levels[49].
GSH is a tri-peptide widely distributed inside living cells. It plays an important role in cell protection from the deleterious effect of oxidative stress, directly and as a cofactor of GSH peroxidase[50]. The inclusion of GSH in cryopreservation extender has had variable results in several species[51,52]. In the present study, concentrations of serum ascorbic acid, reduced glutathione and MDA and NO in seminal plasma were significantly high but age had no significant effect on circulating GHD and MDA and tended to affect its concentrations in seminal plasma. NO plays important roles in sperm physiology by its participation in the regulation of sperm motility and capacitation[53,54].
In absence of peroxidation inducers, only relatively little peroxidation was noted in fresh sperm cells whereas some midpiece peroxidation was noted for frozen-thawed sperm cells that progressed to sperm head leading to loss of head membrane[55].
The exact role of NO in mammalian sperm physiology seems contradictory; low NO-levels are useful, while elevated NO levels seems harmful[56]. Spermatozoa are also capable of producing NO a production that is crucial for sperm motility, capacitation, and fertilization[57,58]. In stallion, the recorded positive correlation between NO production and post-thawing sperm motility and velocity, suggesting a crucial role for maintaining sperm function during cryopreservation[59]. NO has been shown to have detrimental effects on normal sperm functions inhibiting both motility and spermcompetence for zona binding[60].
We concluded that the age of the stallion determinately affected semen variables, oxidant and antioxidant status of either blood or seminal plasma.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgement
The authors are greatly acknowledge the staff and workers at Police Academy stud, Cairo and private stud in Menofya, Egypt providing the logistics support for conducting this research work.
[1] Koppers AJ, De Luliis GN, Finnie JM, McLaughlin EA, Aiteken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa.J Clin Endocrinol Metab2008;93: 3199-207.
[2] Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: The balance of benefit and risk.Bioessays1994;16: 259-267.
[3] Aitken RJ, Marshall GJ. Human spermatozoa: The future of sex.Nature2002;415: 963.
[4] Ball BA, Vo AT, Baumber J. Generation of reactive oxygen species by equine spermatozoa.Am J Vet Res2001;62(4): 508-515.
[5] Almog T, Naor Z. Mitogen activated protein kinases (MAPKs) as regulators of spermatogenesis and spermatozoa functions.Mol Cell Endocrinol2008;282: 39-44.
[6] O’Flaherty CM, Beorlegui NB, Beconi MT. Reactive oxygenpecies requirements for bovine sperm capacitation and acrosome reaction.Theriogenology1999;52: 289-301.
[7] Gordon I.Capacitating bovine sperm. In: Laboratory production of cattle embryos, 2nd ed. Gordon I, CABI Publishing, CAB International. 2003; p158-175.
[8] Sigman M, Zini A. Semen analysis and sperm function assays: What do they mean?Semen Reprod Med2009;27: 115-123.
[9] Aitken RJ, De Luliis GN, Finnie JM, Hedges A, McLachlan R. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: Developmentof diagnostic criteria.Hum Reprod2010;25(10): 2415-2426.
[10] Aurich, C. Factors affecting the plasma membrane function of cooledstored stallion spermatozoa.Anim Reprod Sci2005;89: 65-75.
[11] Brouwers JF, Silva PFN, Gadella BM. New assays for detectionand localization ofendogenous lipid peroxidation products in livingboar sperm after BTS dilution or after freeze–thawing.Theriogenology2005;63: 458-469.
[12] da Silva CM, Macias-Garcia B, Miro-Moran A, Gonzalez-Fernandez L, Morillo-Rodriguez A, Ortega-Ferrusola C, et al. Melatonin reduces lipid peroxidation and apoptotic-like changes in stallion spermatozoa.J Pineal Res2011;51: 172-179.
[13] Vernet P, Aitken RJ, Drevet JR. Antioxidant strategies in the epididymis.Mol Cell Endocrinol2004;216: 31-39.
[14] Ball BA, Gravance CG, Medina V, Baumber J, Liu IK. Catalaseactivity in equine semen.Am J Vet Res2000;61: 1026-1030.
[15] Baumber J, Ball BA. Determination of glutathione peroxidase andsuperoxide dismutase-like activities in equine spermatozoa, seminalplasma, and reproductive tissues.Am J Vet Res2005;66: 1415-1419.
[16] Knight JA, Blaylock RC, Searles DA. The effects of vitamins C and E on lipid peroxidation in stored erythrocytes.Ann Clin Lab Sci1993;23: 51-56.
[17] Hu JH, Tian WQ, Zhao XL, Zan LS, Wang H, Li QW, et al. The cryoprotective effects of ascorbic acid supplementation on bovine semen quality.Anim Reprod Sci2010;121: 72-77.
[18] Halliwell B. Vitamin C: Antioxidant or pro-oxidantin vivo.Free Rad Res1996;25: 439-454.
[19] Aurich JE, Schönherr U, Hoppe H and Aurich C. Effects of antioxidants on motility and membrane integrity of chilled-stored stallion semen.Theriogenology1997;48:185-92.
[20] Morrell JM, Georgakas A, Lundeheim N, Nash D, Davies Morel MCG, Johannisson A. Effect of heterologous and homologous seminal plasma on stallion sperm quality.Theriogenology2014;82: 176-183.
[21] Ball BA. Oxidative stress, osmotic stress and apoptosis: Impact on sperm function and preservation in the horse.Anim Reropd Sci2008;107: 257-267.
[22] Telisman S, Cvitkovic P, Jurasovic J, Pizent A, Gavella M, Rocic B. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men.Environ Health Perspect2000;108: 45-53.
[23] Aydemir B, Kiziler AR, Onaran, Alici B, Ozkara H, Akyolcu MC. Impact of Cu and Fe concentrations on oxidative damage in male infertility.Biol Trace Elem Res2006;112: 193-203.
[24] Massanyi P, Trandzik J, Nad P, Korenekova B, Skalicka M, Toman R, et al. Concentration of copper, iron, zinc, cadmium, lead, and nickel in boar semen and relation to the spermatozoa quality.J Environ Sci Health A Tox Hazard Subst Environ Eng2003;38: 2643-2651.
[25] Massanyi P, Trandzik J, Nad P, Korenekova B, Skalicka M, Toman R, et al. Concentration of copper, iron, zinc, cadmium, lead, and nickel in bull and ram semen and relation to the occurrence of pathologic spermatozoa.J Environ Sci Health A Tox Hazard Subst Environ Eng2004;39: 3005-3014.
[26] Tabassomi M, Alavi-Shoushtari SM. Effects ofin vitrocopper sulphate supplementation on the ejaculated sperm characteristics in water buffaloes (Bubalusbubalis).Vet ResForum2013;4: 31-36.
[27] Cigankova V, Mesaros P, Bires J, Ledecky V, Ciganek J, Tomajkova E.Themorphological structure of the testis in stallions with zinc deficiency.Slovak Vet J1998;23: 97-100.
[28] Barrier-Battut, Dellajarraud H, Legrand E, Bruyas J-F, Fie´ni F, Tainturier D, et al. Calcium, magnesium, copper, and zinc in seminal plasma of fertile stallions, and their relationship with semen freezability.Theriogenology2002;58: 229-232.
[29] Kenney RM, Hurtgen J, Pierson R, Witherspoon D, Simons J. Theriogenology and the equine, Part II, the stallion semen examination.J Soc Theriogenol1983;9: 3-54.
[30] Dowsett KF, Osborne HG, Pattie WA. Morphological characteristics of stallion spermatozoa.Theriogenology1984;22: 463-72.
[31] Beutler F, Duron O, Kelly MB. Improved methods for the determination of blood glutathione.J Lab Clinic Med1963;61: 882-890.
[32] Montgomery HAC, Dymock JF. the determination of nitrite in water.Analyst1961;86: 414-416.
[33] Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction.Anal Biochem1979;95: 351-358.
[34] Harris IJ, Mapson LW, Wang YL. Vitamin method: A simple proteniometrric method for determining ascorbic acid, suitable for use with coloured extracts.Biochem J1942;36(1-2): 183-195.
[35] Ventura S, King EJ. Determination of copper and zinc in blood serum.Biochemical J1951;48(5): lxi-lxii.
[36] SPSS: Statistical package for social science, PC software, Version16, 2007.
[37] Nishikawa Y. Motility and fertilizing ability of frozen horse spermatozoa. In: Proceedings of the 7th international symposium on zootechnology. 1972; 155–167[cited by Amann RP, Cristanelli MJ, Squares EL. Proteins in stallion seminal plasma.J Reprod Fertil Suppl1987;35: 113-120].
[38] Pickett BW, Sullivan JJ, Byers WW, Pace MM, Remmenga EE. Effect of centrifugation and seminal plasma on motility and fertility of stallion and bull spermatozoa.Fertil Steril1975;26: 167-174.
[39] Braun J, Torres-Boggino F, Hochi S, Oguri N. Effect of seminal plasma on motion characteristics of epididymal and ejaculated stallion spermatozoa during storage at 5 ℃.Dtsch Tiera’rztl Wochenschr1994;101: 301-304.
[40] Waheed MM, El-Bahr SM, Al-haider AK. Influence of seminal plasma antioxidants and osteopontin on fertility of the Arabian horse.J Equine Vet Sci2013;3: 705-709.
[41] Cevk M, Tuncer PB, Tafidemr U, Ozurtafi T. comparison of spermatological characteristics and biochemical seminal plasma parameters of normozoospermic and oligoasthenzoospermicbullsof two breeds.Turk J Vet Anim Sci2007;31: 381-387.
[42] Danek J, Wisniewski E, Krumrych W. The dependence between concentrations of zinc, copper, and calcium in blood serum and the characteristics of stallion semen.Medycyna Weterynaryjna1999;55(4): 259-264.
[43] Abou-Shakra FR, Ward NI, Everard DM. The role of trace element in male infertility.Fertil Steril1989;52: 370-310.
[44] Pesch S, Bergmann M, Bostedt H. Determination of some enzymes and macro- and microelements in stallion seminal plasma and their correlations to semen quality.Theriogenology2006;66: 307-313.
[45] Mahsud T, Jamil H, Qureshi ZI, Asi MN, Lodhi LA, Waqas MS, et al. Semen quality parameters and selected bio-chemical constituents level in plasma of Lohi rams.Smalrumi Res2013;113: 175-178.
[46] Colagar AH, Marzony ET. Ascorbic Acid in human seminal plasma: Determination and its relationship to sperm quality.J Clin Biochem Nutr2009;45: 144-149.
[47] Thiele JJ, Friesleben HJ, Fuchs J, Ochsendorf FR. Ascorbic acid and urate in human seminal plasma: determination and interrelationships with chemiluminescence in washed semen.Hum Reprod1995;10(1): 110-115.
[48] Jacob RA, Pianalto FS, Agee RE. Cellular ascorbate depletion in healthy men.J Nutr1992;122: 1111-1118.
[49] Chinoy NJ, Mehta RR, Seethalakshmi L, Sharma JD, Chinoy MR. Effects of vitamin C deficiency on physiology of male reproductive organs of guinea pigs.Int J Fertil1986;31: 232-239.
[50] AtmacaG. Antioxidant effects of sulfur-containing amino acids.Yonsei Med J2004;45(5): 776-788.
[51] Anel-López L, Alvarez-Rodríguez M, García-Álvarez O, Alvarez M, Maroto-Morales A, Anel L, et al. Reduced glutathione and Trolox (vitamin E) as extender supplements in cryopreservation of red deer epididymal spermatozoa.Anim Reprod Sci2012;135(1-4): 37-46.
[52] Câmara DR, Silva SV, Almeida FC, Nunes JF, Guerra MM. Effects of antioxidants and duration of pre-freezing equilibration on frozen-thawed ram semen.Theriogenology2011;76(2): 342-350.
[53] Hellstrom WJ, Bell M, Wang R, Sikka SC. Effect of sodium nitroprusside on sperm motility viability and lipid peroxidation.Fertil Steril1994;61: 1117-1122.
[54] Lewis SEM, Donnelly ET, Sterling ESL, Kennedy MS, Thompson W, Chakravarthy U. Nitric oxide synthase and nitrite production in human spermatozoa: evidence that endogenous nitric oxide is beneficial for sperm motility.Mol Hum Reprod1996;11: 873-878.
[55] Neild DM, Brouwers JF, Colenbrander B, Agüero A, Gadella BM. Lipid peroxide formation in relation to membrane stability of fresh and frozen thawed stallion spermatozoa.Mol Reprod Dev2005;72(2): 230-238.
[56] Vignini A, Nanetti L, Buldreghini E, Moroni C, RicciardoLamonina G, Mantero F, et al. The production of peroxynitrite by human spermatozoa may affect sperm motility through the formation of protein nitrotyrosine.Fertil Steril2006;85: 947-953.
[57] Francavilla F, Santucci R, Macerola B, Ruvolo G, Romano R. Nitric oxide synthase inhibition in human sperm affects sperm-oocyte fusion but not zona pellucida binding.Biol Reprod2000;63: 425–429.
[58] Goud PT, Goud AP, Diamond MP, Gonik MP, Abu Soud H. Nitric oxide extends the oocyte temporal window for optimal fertilization.Free Radic Biol Med2008;45: 453-459.
[59] Ferrusola CO, ´lezFerna´ndez L,GaGarc ´ BM, Salazar-Sandoval C,Rodr ´guez AM, Martinez HR, et al. Effect of cryopreservation on nitric Oxide production by stallion spermatozoa.Bio Reprod2009;81: 1106–1111.
[60] Agarwal A, Makker K, Sharma R. Clinical relevance ofoxidative stress in male factor infertility: An update.Am J Reprod Immunol2008;59: 2-11.
ment heading
10.1016/j.apjr.2016.07.006
*Corresponding author: Amal M. AboEl-maaty, Animal Reproduction and AI department, Veterinary Division, National Research Centre, Dokki, Giza, Egypt
Tel: +202-01221278132
E-mail: amalaboelmaaty1@yahoo.com
 Asian Pacific Journal of Reproduction2016年5期
Asian Pacific Journal of Reproduction2016年5期
- Asian Pacific Journal of Reproduction的其它文章
- Freezability of buffalo semen with TRIS extender enriched with disaccharides (trehalose or sucrose) and different glycerol concentrations
- Profile of peroxidative injury and antioxidant indicators in singleton, twins and multiple bearing goats throughout pregnancy
- Conception rate in Holstein dairy cows having both normal sized follicles and cystic follicles at estrus
- Polymyxin B changes the plasma membrane integrity of cryopreserved bull semen
- Gum arabic improves semen quality and oxidative stress capacity in alloxan induced diabetes rats
- Ovarian hyperstimulation syndrome followed by ovarian torsion in premenopausal patient using adjuvant tamoxifen treatment for breast cancer
